Macro & Micro-Test
Macro & Micro Test, founded in 2010 in Beijing, is a company committed to the R & D, production and sales of new detection technologies and novel in vitro diagnostic reagents based on its self-developed innovative technologies and excellent manufacturing capabilities, supported with professional teams on R & D, production, management and operation. It has passed TUV EN ISO13485:2016, CMD YY/T 0287-2017 IDT IS 13485:2016, GB/T 19001-2016 IDT ISO 9001:2015 and some products CE certification.
300+
products
200+
staff
16000+
square meter
Our Products
To provide first-class medical products and services for mankind, benefit the society and employees.
-
Rapid-test-molecular-platform-Easy-Amp
-
Eudemon™ AIO800 Automatic Molecular Detection System
-
Monkeypox Virus Antigen Detection Kit (Immunochromatography)
-
Dengue Virus, Zika Virus and Chikungunya Virus Multiplex
-
Mycobacterium Tuberculosis Nucleic Acid and Rifampicin,Isoniazid Resistance Detection Kit (Melting Curve)
-
14 High-Risk HPV with 1618 Genotyping Test Kit
-
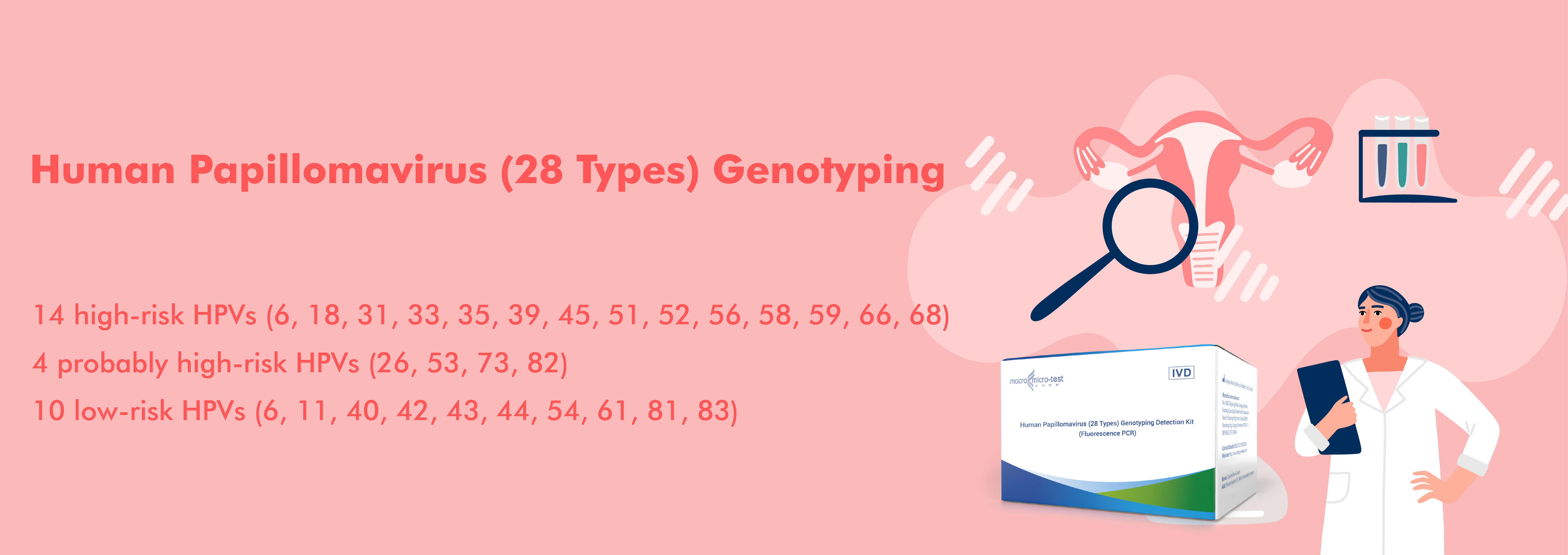
28 Types of HPV Nucleic Acid Detection Kit
-
Nucleic Acid Detection Kit based on Enzymatic Probe Isothermal Amplification (EPIA) for Group B Streptococcus
News
-
Mar 05,26
Measles Resurgence in 2026: When Herd Imm...
At the beginning of 2026, measles has once again become a major global public health concern. Despite being vaccine-preventable, this highly contagious disease is resurging in multiple regions due ...
-
Mar 04,26
International HPV Awareness Day: Turning ...
March 4th is International HPV Awareness Day—a vital opportunity to spotlight one of the most preventable cancers affecting women worldwide: cervical cancer. Despite its global burden, cervical can...
-
Mar 02,26
Unveiling the Rodent “Pathogen Bank” in H...
A recent metagenomic study has revealed a remarkably diverse spectrum of viruses and bacteria carried by rodents in Heilongjiang Province, including Lassa virus, Amur virus, Salmonella, and Yersin...

CONTACT US FOR MORE PRODUCTS
For inquiries about our products or pricelist, please leave your email to us and we will be in touch within 24 hours.