18 Years Factory Dengue Virus Rna - Dengue NS1 Antigen, IgM/IgG Antibody Dual Detection Kit (Immunochromatography) – Macro & Micro-Test
18 Years Factory Dengue Virus Rna - Dengue NS1 Antigen, IgM/IgG Antibody Dual Detection Kit (Immunochromatography) – Macro & Micro-Test Detail:
Product name
Dengue NS1 Antigen, IgM/IgG Antibody Dual Detection Kit (Immunochromatography)
Certificate
CE
Epidemiology
Dengue fever is an acute systemic infectious disease caused by the bite of female mosquitoes carrying dengue virus (DENV), with rapid transmission, high incidence, widespread susceptibility, and high mortality in severe cases[1-2].
Approximately 390 million people worldwide are infected with dengue fever each year, with 96 million people affected by the disease in more than 120 countries, most severely in Africa, the Americas, Southeast Asia and the Western Pacific[3]. As global warming increases, the dengue fever is now spreading to temperate and frigid regions and higher altitudes, and the prevalence of serotypes is changing. In recent years, the epidemic situation of dengue fever is more serious in the South Pacific region, Africa, South America, southern Asia and Southeast Asia, and shows different degrees of increase in its transmission serotype type, altitude area, seasons, mortality rate and number of infections[4-7].
The WHO’s official data in August 2019 showed that there were about 200,000 cases of dengue fever and 958 deaths in Philippines. Malaysia had accumulated more than 85,000 dengue cases in mid-August 2019, while Vietnam had accumulated 88,000 cases. Compared to same period in 2018, the number increased more than twofold in both countries. WHO has considered dengue fever as a major public health problem[8].
This product is a rapid, on-site and accurate detection kit for dengue virus NS1 antigen and IgM/IgG antibody. Specific IgM antibody indicates that there is a recent infection, but a negative IgM test does not prove that the body is not infected. It is also necessary to detect specific IgG antibodies with a longer half-life and the highest content to confirm the diagnosis. In addition, after the body is infected, the NS1 antigen appears first, so the simultaneous detection of the dengue virus NS1 antigen and specific IgM and IgG antibodies can effectively diagnose the body’s immune response to a specific pathogen[9], and this antigen-antibody combined detection kit can perform rapid early diagnosis and screening in the early stage of dengue infection, primary infection and secondary or multiple dengue infection, shorten the window period and improve the detection rate.
Features
● Rapid: Read results within 15 minutes.
● Easy to use: Only 3 steps.
● Convenient: No instrument.
● Room temperature: Transportation & storage at 4-30℃ for 12 months.
● Accuracy: High sensitivity & specificity.
Technical Parameters
| Target region | Dengue virus NS1 antigen, IgM and IgG antibodies |
| Storage temperature | 4℃-30℃ |
| Sample type | Human serum, plasma, venous blood and fingertip blood |
| Shelf life | 12 months |
| Auxiliary instruments | Not required |
| Extra Consumables | Not required |
| Detection time | 15-20 mins |
| Specificity | Conduct the cross-reactivity tests with Japanese encephalitis virus, forest encephalitis virus, hemorrhagic fever with thrombocytopenia syndrome, Xinjiang hemorrhagic fever, hantavirus, hepatitis C virus, influenza A virus, influenza B virus, no cross-reactivity is found. |
Work Flow
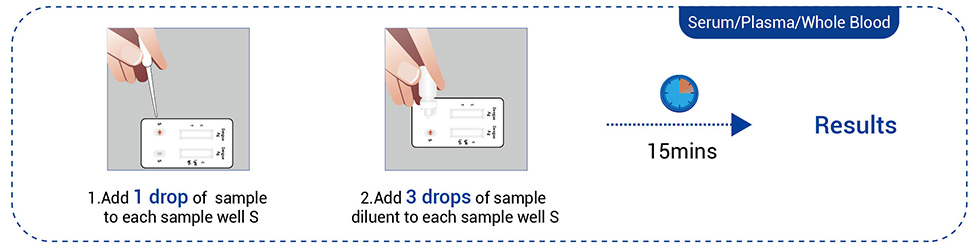
● Venous blood (Serum, Plasma, or Whole blood)
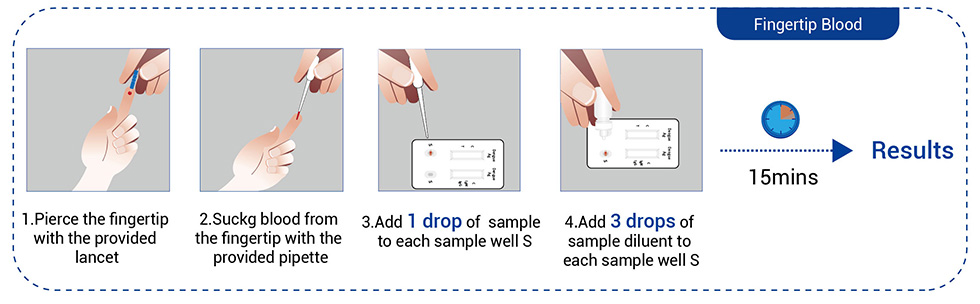
● Fingertip blood
● Read the result (15-20 mins)
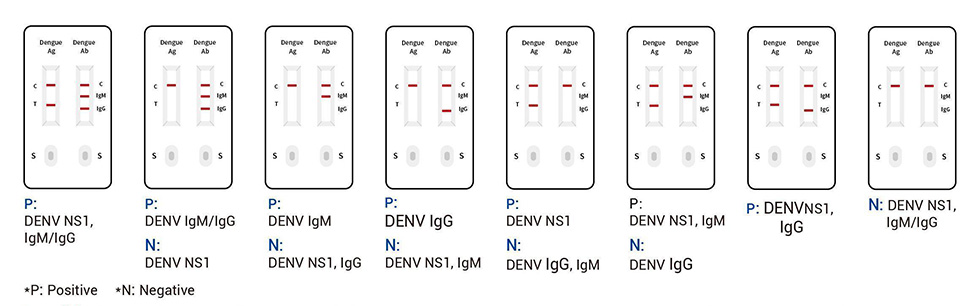
Invaild: If the quality control line “C” is not visible,whatever the test line “T”, “lgM” and “lgG” colored,the result is invaild.
Precautions:
1. Do not read the result after 20 mins.
2. After opening, please use the product within 1 hour.
3. Please add samples and buffers in strict accordance with the instructions.
Main Components
|
Catalogue Number |
Packaging Size | Components | Specification | Quantity | Component Description |
|
HWTS-FE031A |
1 test/kit |
Test Cassette | 1pc/bag | 1 bag | It consists of dengue virus NS1 antigen detection strip and dengue virus IgM/IgG antibody detection strip. The detection strip is composed of nitrocellulose membrane, glass fiber, PVC board and other supports. |
| SampleDiluent | 0.5mL/vial | 1 vial | The sample diluent is mainly PBS. | ||
| Pipette | 1pc/bag | 1 bag | Pipette | ||
| Lancet | 1pc/bag | 1 bag | Lancet | ||
|
HWTS-FE031B |
20 tests/kit | Test Cassette | 1pc/bag | 20 bags | It consists of dengue virus NS1 antigen detection strip and dengue virus IgM/IgG antibody detection strip. The detection strip is composed of nitrocellulose membrane, glass fiber, PVC board and other supports. |
| SampleDiluent | 0.5mL/vial | 20 vials | The sample diluent is mainly PBS. | ||
| Pipette | 20pcs/bag | 1bag | Pipette | ||
| Lancet | 20pcs/bag | 1bag | Lancet |
Reference
[1] Lin Jianyan, Guo Zeqiang, Qiu Changwen. Progress in the study of non-mosquito-borne transmission of dengue virus[J]. China Tropical Medicine, 2018, 18(4):406409.
[2] Ma Haifang. Progress of research on epidemiology of dengue fever [J]. Occupation and Health,2020,36(1):137139,144.
[3] Li Jintao.Advances in dengue fever control research[J]. Journal of Third Military Medical University,2019,41(19):19021907.
[4] INZANC,O’ CONNORO, WORWORG,et al. Molecular characterization of dengue type 2 outbreak in pacific islands countries and territories 20172020[J]. Viruses,2020,12(10):1081.
[5] SINTAYEHFUDW, TASSIEN, DE BOER W F. Present and future climatic suitability for dengue fever in Africa[J].Infect Ecol Epidemiol, 2020,10(1):1782042.
[6] GUTIERREZ-BARBOSA H, MEDINA-MORENO S, ZAPATA JC, et al. Dengue infections in Colombia:epidemiological trends of a hyperendemic country [J].Trop Med Infect Dis, 2020,5 (4): 156.
[7] KALITAJM,AGGARWALA, YEDALEKA, etal. A 5-year study of dengue seropositivity among suspected cases attending a teaching hospital of North-Western region of India [J].J Med Virol, 2020, 93(6) :3338-3343.
[8] National Health and Family Planning Commission of China.Dengue Fever Diagnosis and Treatment Guide(Version 2,2014)[J] Traditional Chinese Drug Research and Clinical
Pharmacology, 2016,27(1):138142.
[9] Shi Yaling, Zhao Rong, Huang Yingyi. Evaluation of clinical application of rapid detection of NS1 antigen and IgG/IgM antibody of dengue fever virus [J]. Laboratory Medicine, 2015, 30(4):363-366.
Product detail pictures:




Related Product Guide:
Sincerity, Innovation, Rigorousness, and Efficiency is definitely the persistent conception of our corporation to the long-term to establish alongside one another with customers for mutual reciprocity and mutual profit for 18 Years Factory Dengue Virus Rna - Dengue NS1 Antigen, IgM/IgG Antibody Dual Detection Kit (Immunochromatography) – Macro & Micro-Test , The product will supply to all over the world, such as: Serbia, Australia, Suriname, We have customers from more than 20 countries and our reputation has been recognized by our esteemed customers. Never-ending improvement and striving for 0% deficiency are our two main quality policies. Should you need anything, don't hesitate to contact us.
A good manufacturers, we have cooperated twice, good quality and good service attitude.


