China OEM Eb Virus Nucleic Acid Detection Kit(Fluorescence Pcr) - EB Virus Nucleic Acid Detection Kit(Fluorescence PCR) – Macro & Micro-Test
China OEM Eb Virus Nucleic Acid Detection Kit(Fluorescence Pcr) - EB Virus Nucleic Acid Detection Kit(Fluorescence PCR) – Macro & Micro-Test Detail:
Product name
EB Virus Nucleic Acid Detection Kit(Fluorescence PCR)
Certificate
CE
Epidemiology
EBV (Epstein-barr virus), or human herpesvirus type 4, is a common human herpesvirus[1]. In recent years, a large number of studies have proved that EBV is associated with occurrence and development of nasopharyngeal cancer, Hodgkin’s disease, T/Natural killer celllymphoma, Burkitt’s lymphoma, breast cancer, gastric cancer and other malignant tumors. And it is also closely associated with post-transplantlymphoproliferative disorders, post-transplant smooth muscle tumor and acquired immunedeficiency syndrome(AIDS) related lymphoma, multiple sclerosis, primary central nervous system lymphoma or leiomyosarcoma[2-4].
Features
● Multiplex PCR Amplification Technology.
● Internal control: Fully monitor the experimental process to ensure the quality of Experiments.
● High sensitivity: 500Copies/mL.
● Convenient: One test for multiple results.
Channel
FAM: EBV
VIC (HEX): Internal control
PCR Amplification Conditions Setting
| Step | Cycles | Temperature | Time | Collect Fluorescent Signals or Not |
| 1 | 1 cycle | 50℃ | 2mins | No |
| 2 | 1 cycle | 95℃ | 5mins | No |
| 3 | 40 cycles | 95℃ | 15s | No |
| 58℃ | 30s | Yes |
Technical Parameters
| Storage | ≤-18℃ In dark |
| Shelf-life | 12 months |
| Specimen Type | Whole blood, Plasma, Serum |
| Ct | ≤38 |
| CV | ≤5.0% |
| LoD | 500Copies/mL |
Specificity: It has no cross-reactivity with other pathogens (such as human herpesvirus 1, 2, 3, 6, 7, 8, hepatitis B virus, cytomegalovirus, influenza A, etc.) or bacteria (Staphylococcus aureus, Candida albicans, etc.)
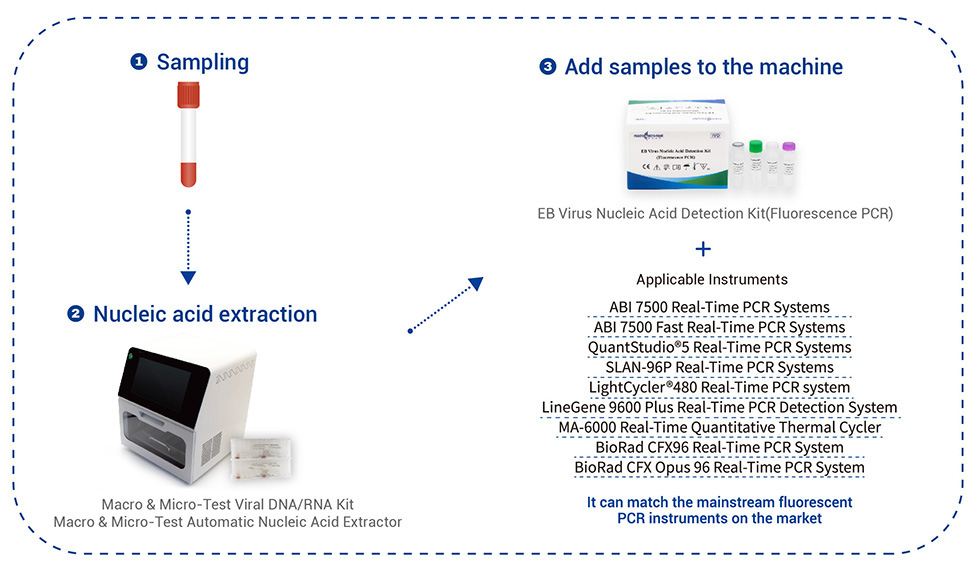
Applicable Instruments: It can match the mainstream fluorescent PCR instruments on the market.
SLAN-96P Real-Time PCR Systems.
ABI 7500 Real-Time PCR Systems.
QuantStudio®5 Real-Time PCR Systems.
LightCycler®480 Real-Time PCR Systems.
LineGene 9600 Plus Real-Time PCR Detection Systems.
MA-6000 Real-Time Quantitative Thermal Cycler.
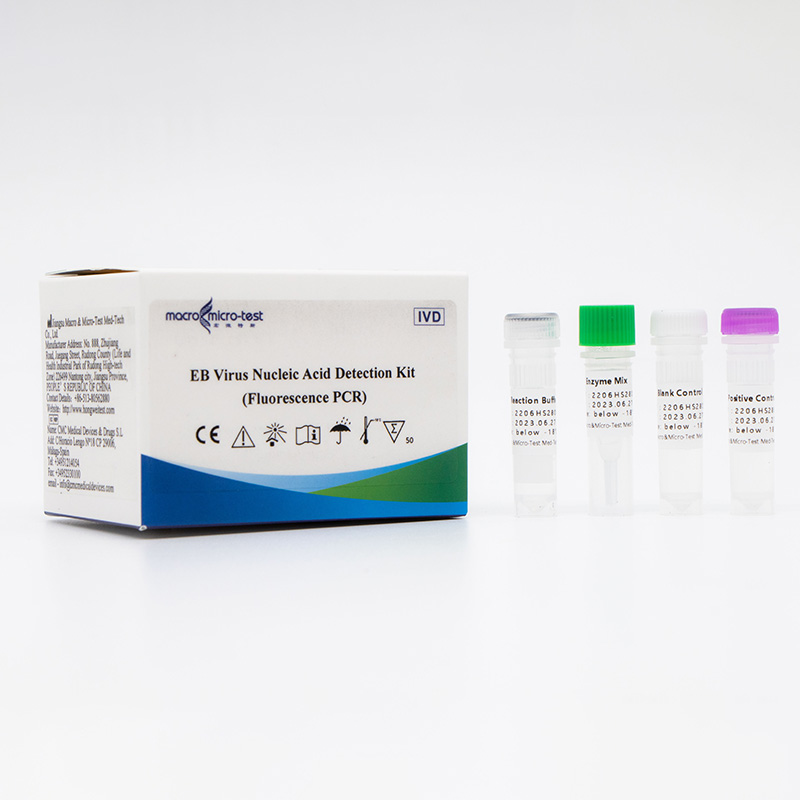
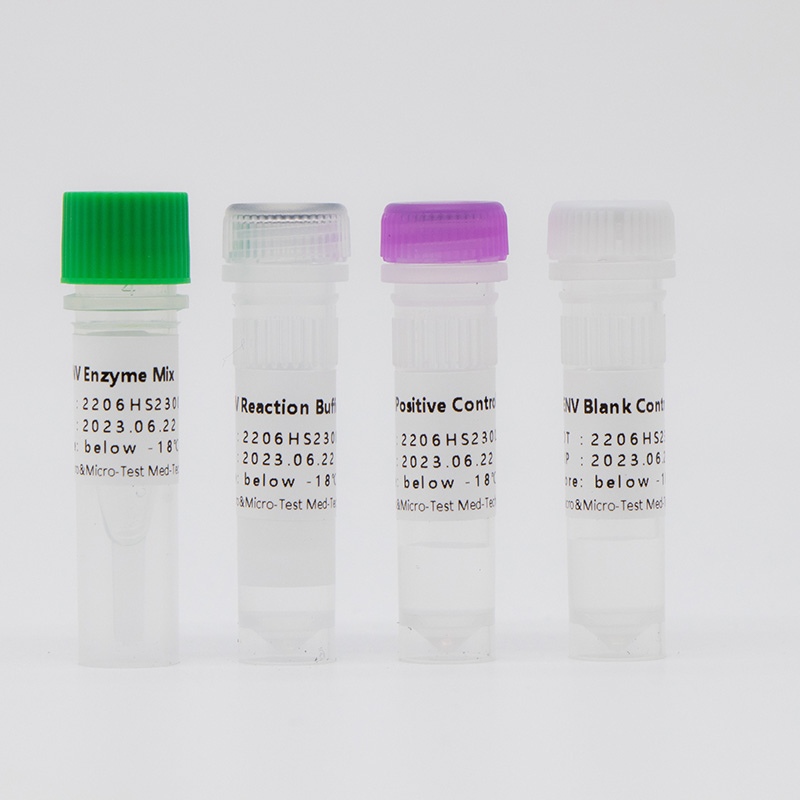
Main Components
| Catalogue Number | Component (50 tests/kit) | Specification | Quantity | Component Description |
| HWTS-OT061A | EB Reaction Buffer | 1.175mL/vial | 1 vial | Amplification reaction reagents, EB virus primer probes, internal reference primer probes, etc. |
| EB Enzyme Mix | 75μL/vial | 1 vial | Taq enzyme, UDG enzyme, etc. | |
| EB Positive Control | 600μL/vial | 1 vial | Mixed solution of viral target gene and internal reference | |
| EB Blank Control | 600μL/vial | 1 vial | DNase/RNase free H2O |
Total PCR Solution
Reference
[1] ohen JI, Jaffe ES, Dale JK, et al. Characterization and treatment of chronic active Epstein-Barr virus disease: a 28-year experience in the United States[J]. Blood, 2011, 117(22): 5835-5849.
[2] Jahangiryan A, Kheirandish M, Samiee S, et al. Determination of Epstein-Barr virus (EBV) incidence in umbilical cord blood (UCB) and assessment of virus DNA via Real-time PCR[J]. Annals of Cancer Research and Therapy, 2021, 29(1):114-120.
[3] Petersson F. Nasopharyngeal carcinoma:a review[J].Semin Diagn Pathol, 2015, 32(1):54-73. [4]Chen J N, He D, Tang F, et al. Epstein-Barr virus-associated gastric carcinoma:a newly defined entity[J]. J Clin Gastroenterol, 2012, 46(4):262-271.
Product detail pictures:


Related Product Guide:
We've numerous great employees customers excellent at promoting, QC, and working with kinds of troublesome difficulty inside the generation method for China OEM Eb Virus Nucleic Acid Detection Kit(Fluorescence Pcr) - EB Virus Nucleic Acid Detection Kit(Fluorescence PCR) – Macro & Micro-Test , The product will supply to all over the world, such as: Liverpool, Japan, Iraq, Selling our products and solutions causes no risks and brings high returns to your company instead. It is our consistent pursuit to create value for clients. Our company is looking for agents sincerely. What are you waiting for? Come and join us. Now or never.
It is not easy to find such a professional and responsible provider in today's time. Hope that we can maintain long-term cooperation.