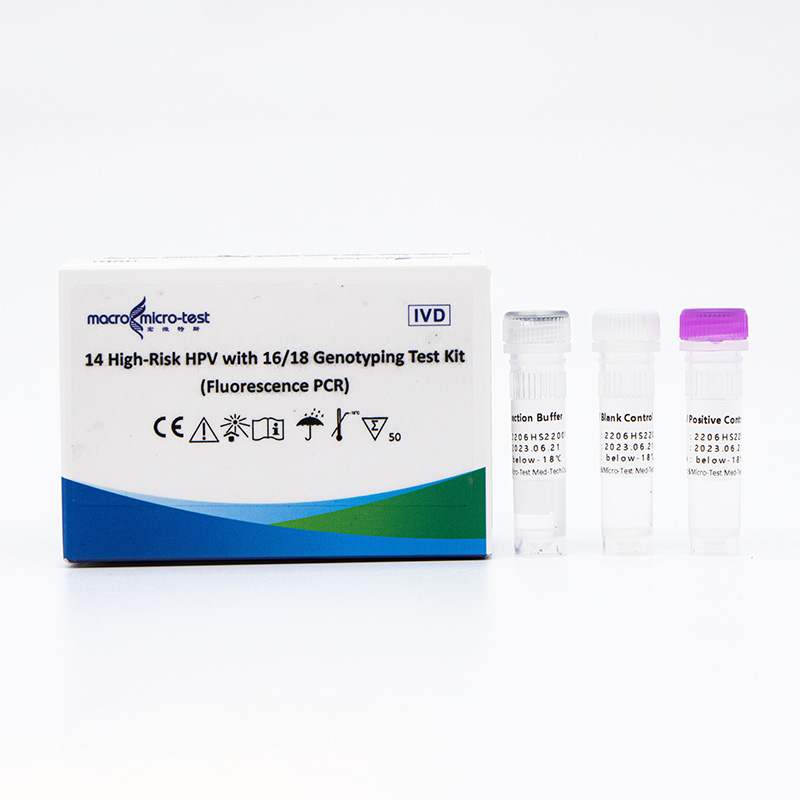
China OEM Hpv Types - 14 High-Risk HPV with 16/18 Genotyping Test Kit (Fluorescence PCR) – Macro & Micro-Test
China OEM Hpv Types - 14 High-Risk HPV with 16/18 Genotyping Test Kit (Fluorescence PCR) – Macro & Micro-Test Detail:
Product name
14 High-Risk HPV with 16/18 Genotyping Test Kit (Fluorescence PCR)
Freeze-dried 14 Types of High-risk Human Papilloma Virus (16/18 Typing) Nucleic Acid Detection Kit (Fluorescence PCR)
Certificate
CE
Epidemiology
Cervical cancer is one of the most common malignant tumors in the female reproductive tract. It has been shown that persistent HPV infection and multiple infections are one of major causes of cervical cancer. Currently there is still a lack of generally accepted effective treatments for cervical cancer caused by HPV. Therefore, early detection and prevention of cervical infection caused by HPV are the keys to prevention of cervical cancerization. Establishment of simple, specific and rapid diagnostic tests for pathogens is of great significance for clinical diagnosis of cervical cancer.
Features
● Multiplex PCR Amplification Technology
● Internal control: Fully monitor the experimental process to ensure the quality of Experiments.
● High sensitivity: 25 Copies/reaction
● High specificity: No cross-reactivity with common respiratory pathogens for more accurate results.
● Convenient: One test for multiple results
Channel
| Channel | Type |
| FAM | HPV 18 |
| VIC/HEX | HPV 16 |
| ROX | HPV 31, 33, 35, 39, 45,51,52, 56, 58, 59, 66, 68 |
| CY5 | Internal Control |
PCR Amplification Conditions Setting
| Step | Cycles | Temperature | Time | Collect Fluorescent Signals or Not |
| 1 | 1 cycle | 50℃ | 2mins | No |
| 2 | 1 cycle | 95℃ | 10mins | No |
| 3 | 10 cycles | 95℃ | 15 s | No |
| 62℃ | 20 s | No | ||
| 4 | 30 cycles | 95℃ | 15 s | No |
| 58℃ | 30 s | Yes |
Technical Parameters
| Storage | ≤-18℃ in dark |
| Shelf-life | 12 months |
| Specimen Type | Cervical exfoliated cells |
| Ct | ≤28 |
| CV | ≤5.0% |
| LoD | Liquid |
| Specificity | No cross-reactivity with common reproductive tract pathogens (such as ureaplasma urealyticum, genital tract chlamydia trachomatis, candida albicans, neisseria gonorrhoeae, trichomonas vaginalis, mold, gardnerella and other HPV types not covered in the kit, etc). |
| Applicable Instruments | It can match the mainstream fluorescent PCR instruments on the market. |
| SLAN-96P Real-Time PCR Systems | |
| ABI 7500 Real-Time PCR Systems | |
| QuantStudio®5 Real-Time PCR Systems | |
| LightCycler®480 Real-Time PCR Systems | |
| LineGene 9600 Plus Real-Time PCR Detection Systems | |
| MA-6000 Real-Time Quantitative Thermal Cycle | |
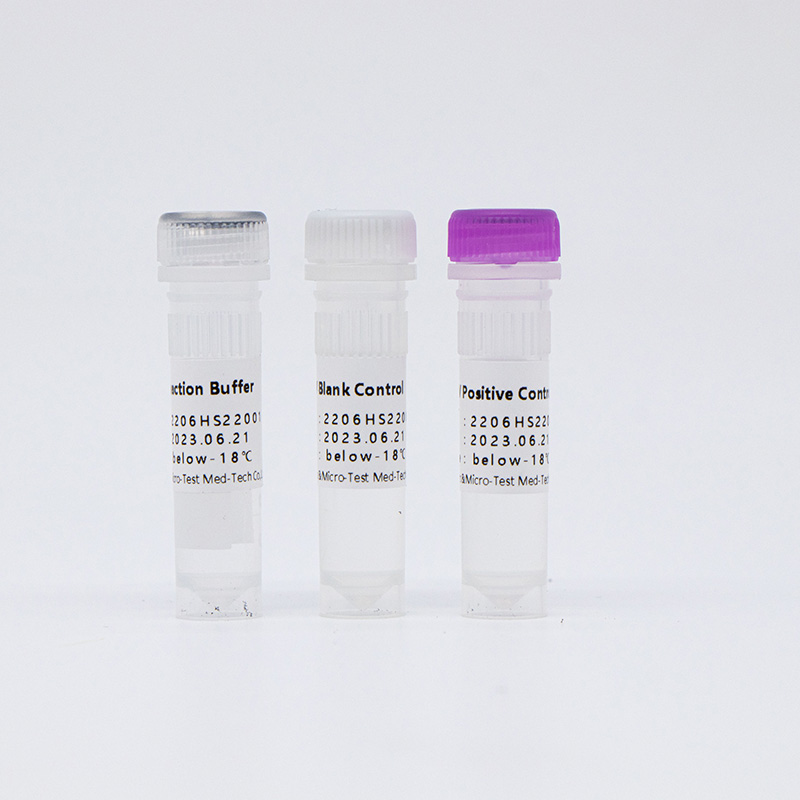
Main Components
14 High-Risk HPV with 16/18 Genotyping Test Kit (Fluorescence PCR)
| Catalogue Number | Component (50 tests/kit) | Specification | Quantity | Component Description |
| HWTS-CC007A | HPV Reaction Buffer | 950μL/vial | 1 vial | Amplification reagents |
| HPV Primer and Probe Mix | 300μL/vial | 1 vial | Mix of various types of primers and probes | |
| HPV Positive Control | 600μL/vial | 1 vial | Mix of HPV 16, 18, 33 and internal control Templates | |
| HPV Blank Control | 600μL/vial | 1 vial | DNase/RNase free H2O |
Freeze-dried 14 Types of High-risk Human Papilloma Virus (16/18 Typing) Nucleic Acid Detection Kit (Fluorescence PCR)
| Component | Character | Catalogue Number | Component Description | |
| HWTS-CC010A | HWTS-CC010B | |||
| Specification/Quantity (20 tests/kit) |
Specification/Quantity (50 tests/kit) |
|||
| HPV Reaction Buffer | Lyophilization | 1 bottle | 1 bottle | Amplification reaction reagents and kinds of primers, probes |
| HPV Positive Control | Lyophilization | 1 vial | 1 vial | Mixture of HPV16, 18, 33 and their internal control plasmids |
| HPV Blank Control | Liquid | 1 vial, 600μL/vial | 1 vial, 600μL/vial | DNase/RNase free H2O |
| Reconstituted Solution | Liquid | 1 vial, 1mL/vial | 1 vial, 1.45mL/vial | DNase/RNase free H2O, etc. |
Total PCR Solution
Option 1.

Option 2.

References
1. Guo Yumei, Sun Jie, Chen Yadan, et al. HPV testing and its significance in the diagnosis of cervical cancer [J]. Chinese Journal of Laboratory Diagnosis, 2009,13: 1306-1308.
2. Li Hua, Gao Guolan. Advance in research on HPV and cervical cancer [J]. The Practical Journal of Cancer, 2007, 22: 420-422.
3. He Guishan. Advance in research on HPV detection methods [J]. Gansu Medical Journal, 2009, 28: 182-183.
4. Ning Tang, Shihai Huang, Brian Erickson, et al. High-risk HPV detection and concurrent HPV 16 and 18 typing with abbott realtime high risk HPV test [J]. Journal of Clinical Virology, 2009, 45: S25-S28.
Product detail pictures:



Related Product Guide:
We take customer-friendly, quality-oriented, integrative, innovative as objectives. Truth and honesty is our administration ideal for China OEM Hpv Types - 14 High-Risk HPV with 16/18 Genotyping Test Kit (Fluorescence PCR) – Macro & Micro-Test , The product will supply to all over the world, such as: Adelaide, Denver, Iraq, we always keep our credit and mutual benefit to our client, insist our high quality service to moving our clients. always welcome the our friends and clients to come and visit our company and guid our business, if you are interested in our products, you can also submit your purchase information online, and we will contact you immediately, we keep our highly sincere cooperation and wish everything in your side are all well.
We have worked with many companies, but this time is the best,detailed explanation, timely delivery and quality qualified, nice!