Dengue Virus IgM/IgG Antibody
Product name
HWTS-FE030-Dengue Virus IgM/IgG Antibody Detection Kit (Immunochromatography)
Certificate
CE
Epidemiology
This product is suitable for the qualitative detection of dengue virus antibodies, including IgM and IgG, in human serum, plasma and whole blood samples.
Dengue fever is an acute infectious disease caused by dengue virus, and it is also one of the most widely spread mosquito-borne infectious diseases in the world. Serologically, it is divided into four serotypes, DENV-1, DENV-2, DENV-3, and DENV-4[1]. Dengue virus can cause a series of clinical symptoms. Clinically, the main symptoms are sudden high fever, extensive bleeding, severe muscle pain and joint pain, extreme fatigue, etc., and are often accompanied by rash, lymphadenopathy and leukopenia[2]. With the increasingly serious global warming, the geographical distribution of dengue fever tends to spread, and the incidence and severity of the epidemic also increase. Dengue fever has become a serious global public health problem.
This product is a rapid, on-site and accurate detection kit for dengue virus antibody (IgM/IgG). If it is positive for IgM antibody, it indicates a recent infection. If it is positive for IgG antibody, it indicates a longer infection time or previous infection. In patients with primary infection, IgM antibodies can be detected 3-5 days after the onset, and peak after 2 weeks, and can be maintained for 2-3 months; IgG antibodies can be detected 1 week after the onset, and IgG antibodies can be maintained for several years or even whole life. Within 1 week, If the detection of a high level of specific IgG antibody in the serum of the patient within one week of the onset, it indicates a secondary infection, and a comprehensive judgment can also be made in combination with the ratio of IgM/IgG antibody detected by the capture method. This method can be used as a supplement to viral nucleic acid detection methods.
Technical Parameters
| Target region | Dengue IgM and IgG |
| Storage temperature | 4℃-30℃ |
| Sample type | human serum, plasma, venous blood and peripheral blood, including blood samples containing clinical anticoagulants (EDTA, heparin, citrate). |
| Shelf life | 24 months |
| Auxiliary instruments | Not required |
| Extra Consumables | Not required |
| Detection time | 15-20 mins |
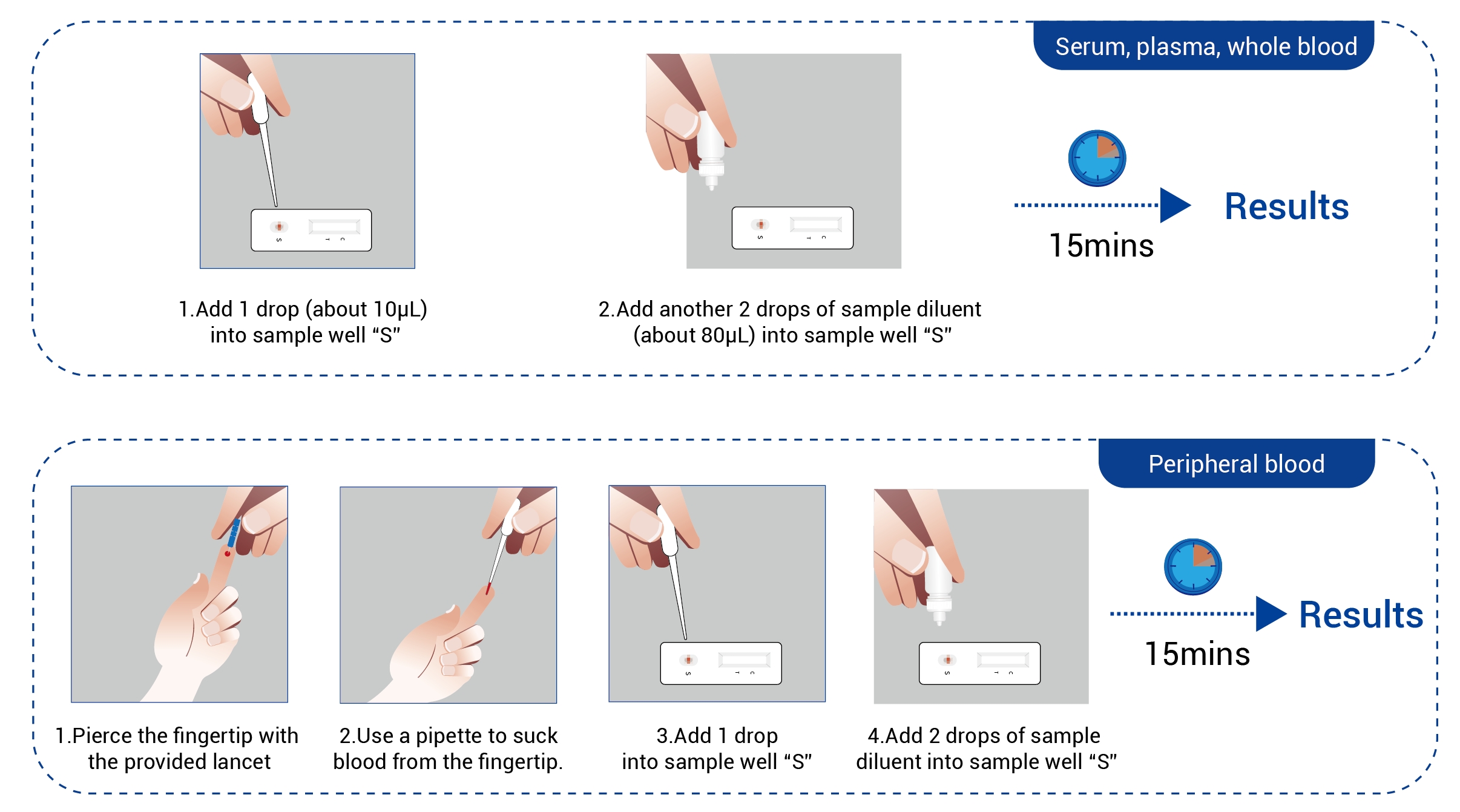
Work Flow