Factory Supply Malaria Pf Ag Detection Kit - Plasmodium Falciparum/Plasmodium Vivax Antigen Detection Kit(Colloidal Gold) – Macro & Micro-Test
Factory Supply Malaria Pf Ag Detection Kit - Plasmodium Falciparum/Plasmodium Vivax Antigen Detection Kit(Colloidal Gold) – Macro & Micro-Test Detail:
Product name
Plasmodium Falciparum/Plasmodium Vivax Antigen Detection Kit(Colloidal Gold)
Certificate
CE
Epidemiology
Malaria (Mal for short) is caused by Plasmodium, which is a single-celled eukaryotic organism, including Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae Laveran, and Plasmodium ovale Stephens. It is a mosquito-borne and blood-borne parasitic disease that seriously endangers human health. Of the parasites that cause malaria in humans, Plasmodium falciparum is the deadliest and is most common in sub-Saharan Africa and causes most malaria deaths globally. Plasmodium vivax is the predominant malaria parasite in most countries outside sub-Saharan Africa.
Features
● Rapid: Read results within 15 minutes.
● Easy to use: Only 3 steps.
● Convenient: No instrument.
● Room temperature: Transportation & storage at 4-30℃ for 24 months.
● Accuracy: High sensitivity & specificity.
Technical Parameters
| Target region | Plasmodium falciparum and Plasmodium vivax |
| Storage temperature | 4-30 ℃ sealed dry storage |
| Sample type | Human peripheral blood and venous blood. |
| Shelf life | 24 months |
| Auxiliary instruments | Not required |
| Extra Consumables | Not required |
| Detection time | 15-20 mins |
| Specificity | There is no cross-reactivity with influenza A H1N1 virus, H3N2 influenza virus, influenza B virus, dengue fever virus, encephalitis B virus, respiratory syncytial virus, meningococcus, parainfluenza virus, rhinovirus, toxic bacillary dysentery, staphylococcus aureus, escherichia coli, streptococcus pneumoniae or klebsiella pneumoniae, salmonella typhi, and rickettsia tsutsugamushi, and the test results are all negative. |
Work Flow
1. Sampling
● Clean the fingertip with an alcohol pad.
● Squeeze the end of the fingertip and pierce it with the provided lancet.

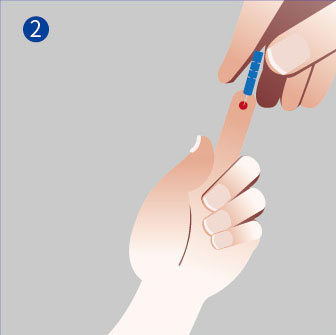
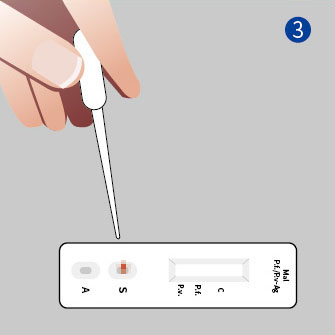
2. Add the sample and solution
● Add 1 drop of sample to the “S” well of the cassette.
● Hold the buffer bottle vertically, and drop 3 drops (about 100 μL) into the “A” well.
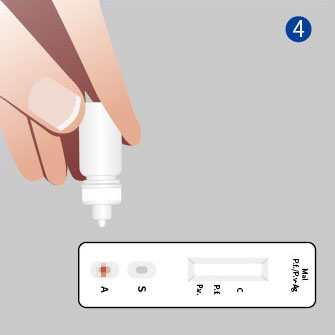
3. Read the result (15-20mins)
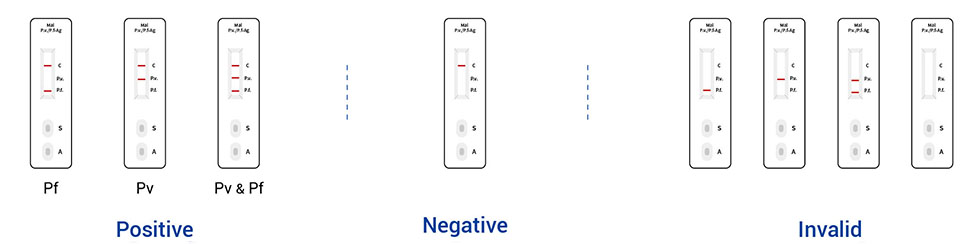
*Pf: Plasmodium falciparum Pv: Plasmodium vivax
Precautions:
1. Do not read the result after 20 mins.
2. After opening, please use the product within 1 hour.
3. Please add samples and buffers in strict accordance with the instructions.
Main Components
|
Catalogue Number |
Packaging Size | Components | Specification | Quantity |
|
HWTS-OT055A |
1 test/kit | Test Cassette | 1pc/bag | 1 bag |
| Sample Extraction Solution | 0.2mL/bottle | 1 bottle | ||
| Lancet | 1pc/bag | 1 bag | ||
| Pipette | 1pc/bag | 1 bag | ||
|
HWTS-OT055B |
20 tests/kit | Test Cassette | 1pc/bag | 20 bags |
| Sample Extraction Solution | 4mL/bottle | 1 bottle | ||
| Lancet | 20pcs/bag | 1 bag | ||
| Pipette | 20pcs/bag | 1 bag |
Product detail pictures:



Related Product Guide:
It really is our obligation to satisfy your requirements and efficiently serve you. Your fulfillment is our greatest reward. We're hunting forward to your check out for joint development for Factory Supply Malaria Pf Ag Detection Kit - Plasmodium Falciparum/Plasmodium Vivax Antigen Detection Kit(Colloidal Gold) – Macro & Micro-Test , The product will supply to all over the world, such as: Latvia, Turkmenistan, Manila, We follow up the career and aspiration of our elder generation, and we're eager to open up a new prospect in this field, We insist on Integrity, Profession, Win-win Cooperation, because we have now a strong backup, that are excellent partners with advanced manufacturing lines, abundant technical strength, standard inspection system and good production capacity.
Product variety is complete, good quality and inexpensive, the delivery is fast and transport is security, very good, we are happy to cooperate with a reputable company!