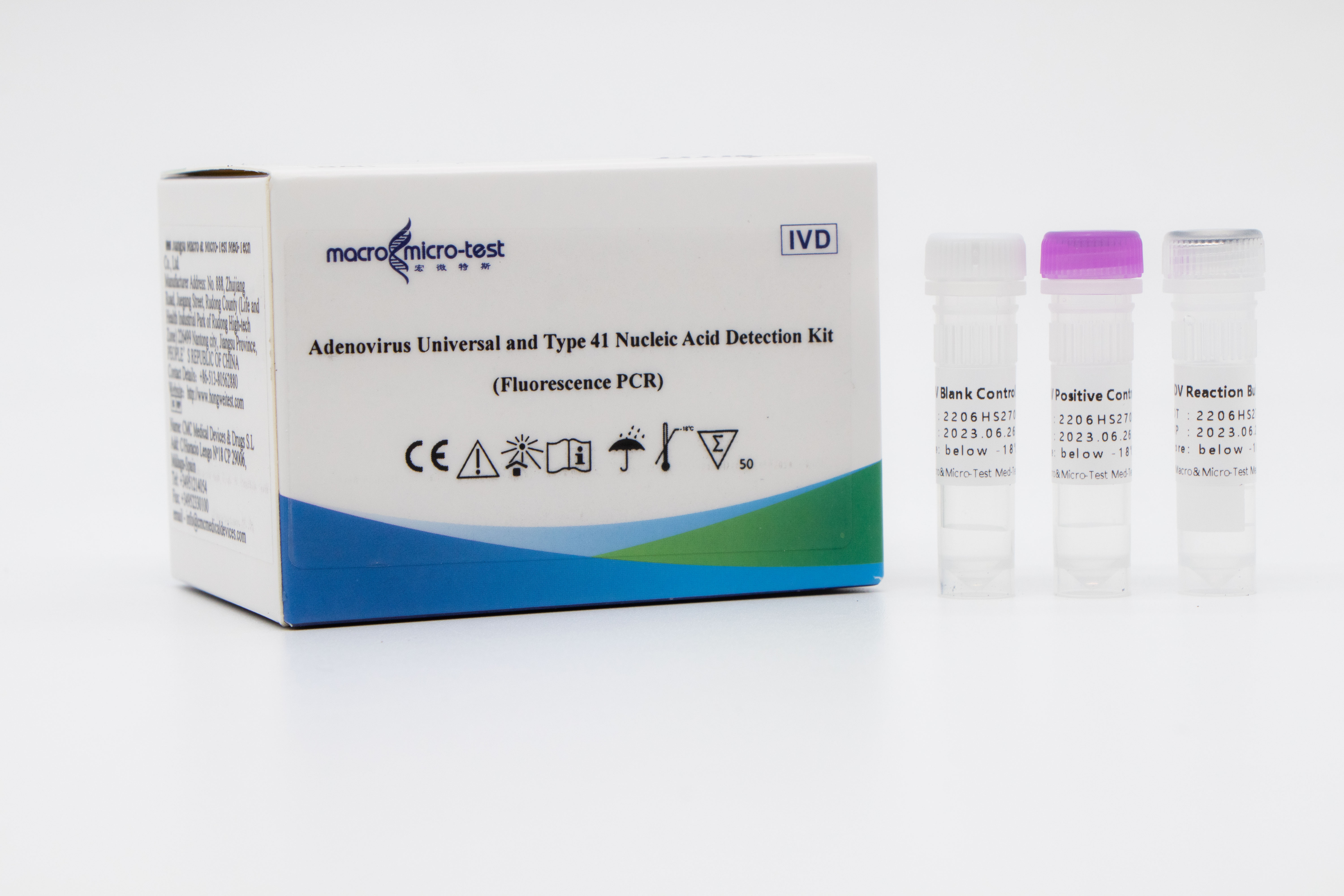
Factory wholesale Real Time Fluorescent Rt-Pcr Kit For Detecting Six Kinds Of Respiratory Pathogens - AdV Universal and Type 41 Nucleic Acid Detection Kit (Fluorescence PCR) – Macro & Micro-Test
Factory wholesale Real Time Fluorescent Rt-Pcr Kit For Detecting Six Kinds Of Respiratory Pathogens - AdV Universal and Type 41 Nucleic Acid Detection Kit (Fluorescence PCR) – Macro & Micro-Test Detail:
Product name
Adenovirus Universal and Type 41 Nucleic Acid Detection Kit (Fluorescence PCR)
Certificate
CE
Packaging Size
1. Liquid product: 50 tests/kit
2. Freeze-dried product (penicillin vial): 20 tests/kit, 50 tests/kit
3. Freeze-dried product (8-tube strips): 48 tests/kit
Intended Use
This kit is used for in vitro qualitative detection of adenovirus nucleic acid in nasopharyngeal swabs, throat swabs and stool samples.
Epidemiology
Human adenovirus (HAdV) belongs to the genus Mammalian adenovirus, which is a double-stranded DNA virus without envelope. Adenoviruses that have been found so far include 7 subgroups (A-G) and 67 types, of which 55 serotypes are pathogenic to humans. Among them, could leading to the respiratory tract infections are mainly group B (Types 3, 7, 11, 14, 16, 21, 50, 55), Group C (Types 1, 2, 5, 6, 57) and Group E (Type 4), and could leading to intestinal diarrhea infection is Group F (Types 40 and 41)[1-8].
Respiratory diseases caused by respiratory tract infections of human body account for 5%~15% of global respiratory diseases, and 5%~7% of global childhood respiratory diseases[9], which could also infect the gastrointestinal tract, urethra, bladder, eyes, and liver, etc. Adenovirus is endemic in a wide range of areas and can be infected all year round, especially in crowded areas, which are prone to local outbreaks, mainly in schools and military camps.
Features
Multiplex PCR Amplification Technology
Internal control: Fully monitor the experimental process to ensure the quality of Experiments.
High sensitivity: 300Copies/mL
High specificity: No cross-reactivity with common respiratory pathogens for more accurate results.
Rapid: Rapid PCR amplification, test results can be obtained in 40 minutes.
Channel
FAM: Adenovirus universal nucleic acid
ROX: Adenovirus type 41 nucleic acid
VIC (HEX): Internal control
PCR Amplification Conditions Setting
| Step | Cycles | Temperature | Time | Collect Fluorescent Signals or Not |
| 1 | 1 cycle | 50℃ | 2mins | No |
| 2 | 1 cycle | 95℃ | 1min | No |
| 3 | 40 cycles | 95℃ | 5s | No |
| 55℃ | 15s | Yes |
Technical Parameters
Storage:
Liquid: ≤-18℃ In dark
Lyophilization: ≤30℃ In dark
Shelf-life: 12 months
Specimen Type: Nasopharyngeal swab, Throat swab, Stool samples
Ct: ≤38
CV: ≤5.0%
LoD: 300Copies/mL
Specificity: Use this kit to detect and there is no cross-reactivity with other respiratory pathogens (such as Influenza A virus, Influenza B virus, Respiratory syncytial virus, Parainfluenza virus, Rhinovirus, Human metapneumovirus, etc.) or bacteria (Streptococcus pneumoniae, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii, Staphylococcus aureus, etc.) and common gastrointestinal pathogens Group A rotavirus, Escherichia coli, etc.
Applicable Instruments: It can match the mainstream fluorescent PCR instruments on the market.
ABI 7500 Real-Time PCR Systems
ABI 7500 Fast Real-Time PCR Systems
SLAN-96P Real-Time PCR Systems
QuantStudio®5 Real-Time PCR Systems
LightCycler®480 Real-Time PCR Systems
LineGene 9600 Plus Real-Time PCR Detection Systems
MA-6000 Real-Time Quantitative Thermal Cycler
Main Components
Liquid product
| Catalogue Number | Component (50 tests/kit) | Specification | Quantity | Component Description |
| HWTS-RT112A | ADV Reaction Buffer | 1.25mL /vial | 1 vial | Amplification reaction reagent, adenovirus universal primer probe, adenovirus type 41 primer probe, internal control primer probe, etc. |
| ADV Positive Control | 250μL/vial | 1 vial | Adenovirus and internal control plasmids mixture. | |
| ADV Blank Control | 250μL/vial | 1 vial | DNase/RNase free H2O |
Lyophilized product
| Component | Character | Catalogue Number | Component Description | ||
| HWTS-RT112B | HWTS-RT112C | HWTS-RT112Z | |||
| Specification/Quantity
(20 tests/kit) |
Specification/Quantity
(50 tests/kit) |
Specification/Quantity
(48 tests/kit) |
|||
| ADV Reaction Buffer | lyophilized | 1 bottle | 1 bottle | 8-tube strips, 6 strips | Amplification reaction reagent, adenovirus universal primer probe, adenovirus type 41 primer probe, internal control primer probe, etc. |
| ADV Positive Control | lyophilized | 1 vial | 1 vial | 1 vial | Adenovirus and internal control plasmids mixture. |
| ADV Blank Control | Liquid | 1 vial, 250μL/vial | 1 vial, 250μL/vial | 1 vial, 250μL/vial | DNase/RNase free H2O |
| ADV Reconstituted Solution | Liquid | 1 vial, 650μL/vial | 1 vial, 1.25mL/vial | 1 vial, 1.21mL/vial | DNase/RNase free H2O, etc. |
Total PCR Solution
Option 1.

Option 2.

Reference
[1] SunB, HeH, WangZ, et al. Emergent severe acute respiratory distress syndrome caused by adenovirus type 55 in immunocompetent adults in 2013: a prospective observational study[J]. Crit Care, 2014, 18(4):456.
[2] CaoB, HuangGH, PuZH, et al. Emergence of community-acquired adenovirus type 55 as a cause of community-onset pneumonia[J]. Chest, 2014, 145(1):79-86.
[3] ABBAS K Z, LOMBOS E, DUVVURI V R, et al. Temporal changes in respiratory adenovirus serotypes circulating in the greater Toronto area, Ontario, during December 2008 to April 2010[J]. Virology Journal, 2013, 10(1): 15. DOI: 10.1186/1743-422X-10-15.
[4] YEUNG R, ESHAGHI A, LOMBOS E, et al. Characterization of culture-positive adenovirus serotypes from respiratory specimens in Toronto, Ontario, Canada: September 2007- June 2008[J]. Virology Journal, 2009,6(1): 11. DOI: 10.1186/1743-422x-6-11.
[5] NODA M, YOSHIDA T, SAKAGUCHI T, et al. Molecular and epidemiological analyses of human adenovirus type 7 strains isolated from the 1995 nation wide outbreak in Japan [J]. Journal of Clinical Microbiology, 2002, 40(1): 140-145.
[6] BARRAZA E M, LUDWIG S L, GAYDOS J C, et al. Reemergence of Adenovirus Type 4 Acute Respiratory Disease in Military Trainees: Report of an Outbreak during a Lapse in Vaccination[J]. Journal of Infectious Diseases, 1999, 179(6): 1531-1533.
[7] MEI Y F, SKOG J, LINDMAN K, et al. Comparative analysis of the genome organization of human adenovirus 11, a member of the human adenovirus species B, and the commonly used human adenovirus 5 vector, a member of species C[J]. Journal of General Virology, 2003, 84(8): 2061-2071.
[8] PRIMO D, PACHECO G T, TIMENETSKY M. Surveillance and molecular characterization of human adenovirus in patients with acute gastroenteritis in the era of rotavirus vaccine, Brazil, 2012-2017[J]. Journal of Clinical Virology, 2018, 109: 35-40.
[9] Ghebremedhin B. Human adenovirus: Viral pathogen with increasing importance[J]. European Journal of Microbiology and Immunology, 2014, 4(1): 26-33.





Product detail pictures:




Related Product Guide:
Our enterprise since its inception, often regards solution excellent as enterprise life, continually strengthen output technology, enhance product high quality and continually strengthen organization total high-quality administration, in strict accordance using the national standard ISO 9001:2000 for Factory wholesale Real Time Fluorescent Rt-Pcr Kit For Detecting Six Kinds Of Respiratory Pathogens - AdV Universal and Type 41 Nucleic Acid Detection Kit (Fluorescence PCR) – Macro & Micro-Test , The product will supply to all over the world, such as: Malaysia, Czech Republic, Mali, Our company always provides good quality and reasonable price for our customers. In our efforts, we already have many shops in Guangzhou and our products have won praise from customers worldwide. Our mission has always been simple: To delight our customers with best quality hair products and deliver on time. Welcome new and old customers to contact us for the future long term business relationships.
This manufacturer can keep improving and perfecting products and service, it is in line with the rules of market competition, a competitive company.