Freeze-dried Influenza Virus/Influenza B Virus Nucleic Acid
Product name
HWTS-RT193-Freeze-dried Influenza Virus/Influenza B Virus Nucleic Acid Detection Kit (Fluorescence PCR)
Epidemiology
According to the antigenic differences between NP and M genes, influenza viruses can be divided into four types: influenza A virus (IFV A), influenza B virus (IFV B), influenza C virus (IFV C) and influenza D virus (IFV D) . For influenza A virus, it has many hosts and complex serotypes, and can spread across hosts through genetic recombination and adaptive mutations. Humans lack lasting immunity to influenza A virus, so people of all ages are generally susceptible. Influenza A virus is the most major pathogens causing influenza pandemics. For influenza B virus, it mostly spreads in small areas and currently has no subtypes. The human infections are mainly caused by influenza viruses of B/Yamagata or B/Victoria lineages. Among the monthly confirmed cases of influenza in 15 countries of the Asia-Pacific region, the diagnosis rate of influenza B virus ranges from 0 to 92%. Unlike the influenza A virus, certain groups of people, such as children and the elderly, are susceptible to influenza B virus, which can easily cause complications, posing even more burdens to society than influenza A virus.
Technical Parameters
| Storage | 2-28℃ |
| Shelf-life | 12 months |
| Specimen Type | Throat swab |
| Ct | IFV A,IFVB Ct≤35 |
| CV | <5.0% |
| LoD | 200 Copies/mL |
| Specificity | Cross-reactivity: There is no cross-reactivity between the kit and Bocavirus, Rhinovirus, Cytomegalovirus, Respiratory syncytial virus, Parainfluenza virus, Epstein-Barr virus, herpes simplex virus, varicella-zoster virus, Mumps virus, Enterovirus, Measles virus, human metapneumovirus, Adenovirus, human coronaviruses, novel coronavirus, SARS-CoV, MERS-CoV, Rotavirus, Norovirus, Chlamydia pneumoniae, Mycoplasma pneumoniae, Streptococcus pneumoniae, Klebsiella pneumoniae, Streptococcus pyogenes, Legionella, Pneumocystis jirovecii, Haemophilus influenzae, Bordetella pertussis, Staphylococcus aureus, Mycobacterium tuberculosis, Neisseria gonorrhoeae, Candida albicans, Candida glabrata, Aspergillus fumigatus, Cryptococcus neoformans, Streptococcus salivarius, Moraxella catarrhalis, Lactobacillus, Corynebacterium and human genomic DNA.
Interference test: Mucin (60mg/mL), human blood (50%), Phenylephrine (2 mg/mL), Oxymetazoline (2mg/mL), Sodium chloride (20mg/mL) with 5% preservative, Beclomethasone (20mg/mL), Dexamethasone (20mg/mL), Flunisolide (20μg/mL), Triamcinolone (2mg/mL), Budesonide (1mg/mL), Mometasone (2mg/mL), Fluticasone (2mg/mL), Histamine hydrochloride (5 mg/mL), Benzocaine (10%), Menthol (10%), Zanamivir (20mg/mL), Peramivir (1mg/mL), Mupirocin (20mg/mL), Tobramycin (0.6mg/mL), Oseltamivir (60ng/mL), Ribavirin (10mg/L) were selected for the interference test, and the results showed that the interfering substances at the above concentrations had no interference reaction to the test results of the kit. |
| Applicable Instruments | Applicable to Type I test reagent:
Applied Biosystems 7500 Real-Time PCR Systems, SLAN-96P Real-Time PCR Systems (Hongshi Medical Technology Co., Ltd.). Applicable to Type II test reagent: EudemonTM AIO800 (HWTS-EQ007) by Jiangsu Macro & Micro-Test Med-Tech Co., Ltd. |
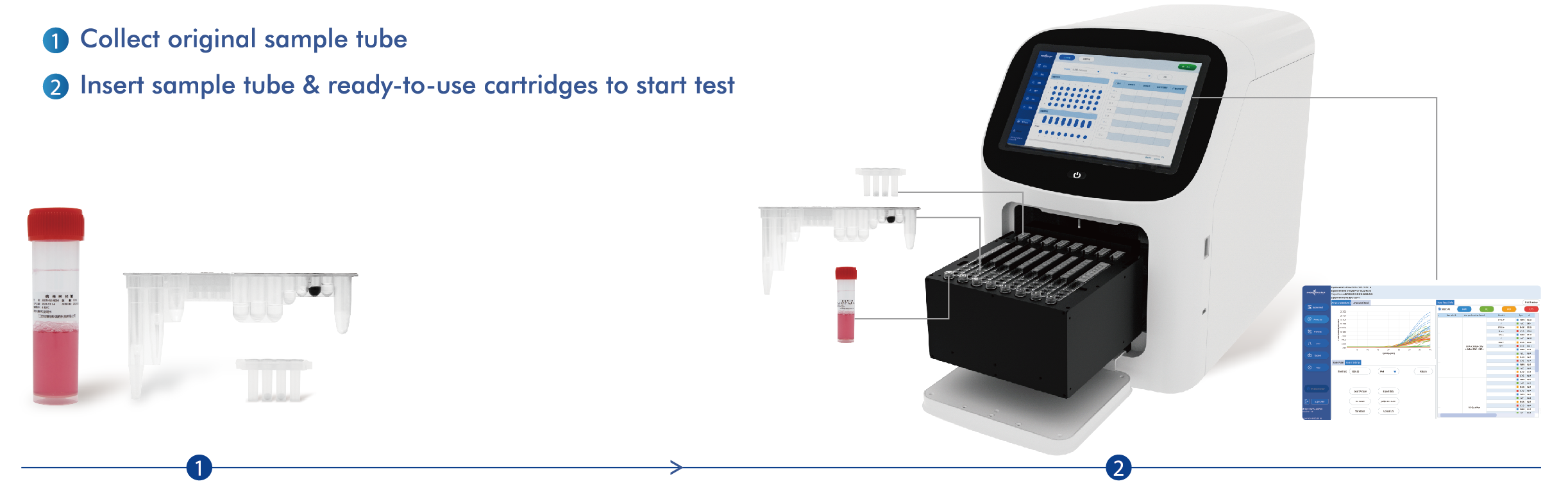
Work Flow
Conventional PCR
Macro & Micro-Test General DNA/RNA Kit (HWTS-3019) (which can be used with Macro & Micro-Test Automatic Nucleic Acid Extractor (HWTS-3006C, (HWTS-3006B) by Jiangsu Macro & Micro-Test Med-Tech Co., Ltd. are recommended for the sample extraction and the subsequent steps should be conducted in strict accordance with the IFU of the Kit.
AIO800 all-in-one machine