Good Quality Covid-19 - SARS-CoV-2 Virus Antigen Detection Kit (colloidal gold)-Home test – Macro & Micro-Test
Good Quality Covid-19 - SARS-CoV-2 Virus Antigen Detection Kit (colloidal gold)-Home test – Macro & Micro-Test Detail:
Product name
SARS-CoV-2 Virus Antigen Detection Kit (colloidal gold method)-Nasal
Certificate
CE1434
Epidemiology
Coronavirus Disease 2019(COVID-19), is a pneumonia caused by infection with a novel coronavirus named as Severe Acute Respiratory Syndrome Corona-Virus 2 (SARS-CoV-2). SARS-CoV-2 is a novel coronavirus in β genus, enveloped particles in round or oval, with a diameter from 60 nm to 140 nm. Human is generally susceptible to SARS-CoV-2. The main sources of infection are the confirmed COVID-19 patients and asymptomatic carrier of SARSCoV-2[1].
Features
● Rapid: Read results in 15 minutes.
● Easy to use: Only 3 steps.
● Convenient: For self-test, home test.
● Room temperature: Transportation & storage at 4-30℃ for 24 months.
● Accuracy: High sensitivity & specificity.
Clinical study
The performance of Antigen Detection Kit was evaluated in 554 patients of nasal swabs collected from symptomatic suspects of COVID-19 within 7 days post symptom onset compared to RT-PCR assay. The performance of the SARS-CoV-2 Ag Test Kit is as follows:
| SARS-CoV-2 Virus Antigen (investigational reagent) | RT-PCR reagent | Total | |
| Positive | Negative | ||
| Positive | 97 | 0 | 97 |
| Negative | 7 | 450 | 457 |
| Total | 104 | 450 | 554 |
| Sensitivity | 93.27% | 95.0% CI | 86.62% – 97.25% |
| Specificity | 100.00% | 95.0% CI | 99.18% – 100.00% |
| Total | 98.74% | 95.0% CI | 97.41% – 99.49% |
Technical Parameters
| Storage temperature | 4℃-30℃ |
| Sample type | Nasal swab samples |
| Shelf life | 24 months |
| Auxiliary instruments | Not required |
| Extra Consumables | Not required |
| Detection time | 15-20 mins |
| Specificity | There is no cross-reactivity with pathogens such as human Coronavirus ( HCoV-OC43, HCoV-229E, HCoV-HKU1, HCoV-NL63), Novel influenza A H1N1 (2009), seasonal influenza A (H1N1, H3N2, H5N1, H7N9), Influenza B (Yamagata, Victoria), Respiratory syncytial virus A/B, Parainfluenza virus(1, 2 and 3), Rhinovirus (A, B, C), Adenovirus (1, 2, 3, 4,5, 7, 55). |
Work Flow
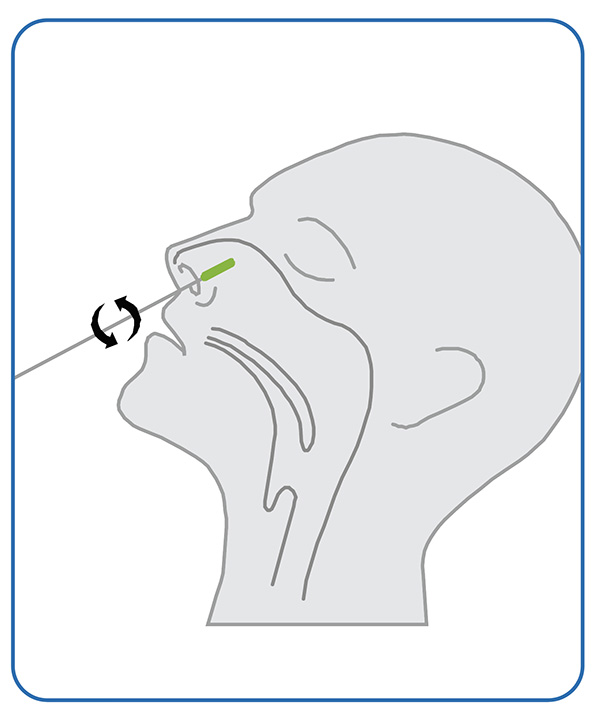
1. Sampling
● Gently insert the entire soft tip of the swab (usually 1/2 to 3/4 of an inch) into one nostril, Using medium pressure, rub the swab against all the inside walls of your nostril. Make at least 5 big circles. And each nostril must be swabbed for about 15 seconds.Using the same swab, repeat the same in your other nostril.
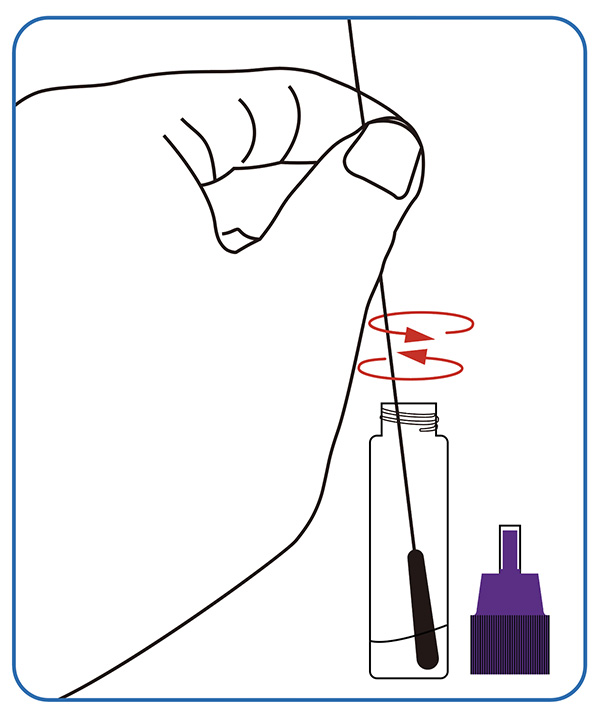
● Sample dissolving. Dip the swab completely into the sample extraction solution; Break the swab stick at the breaking point, leaving the soft end in the tube. Screw on the cap, invert 10 times and put the tube at a stable place.
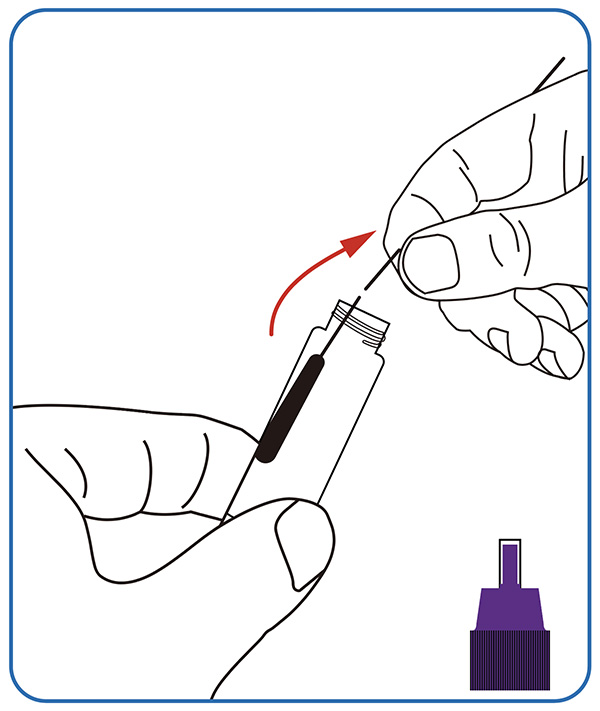
2. Perform the test
Put 3 drops of the processed extracted sample into the sample hole of the detection card, screw the cap.
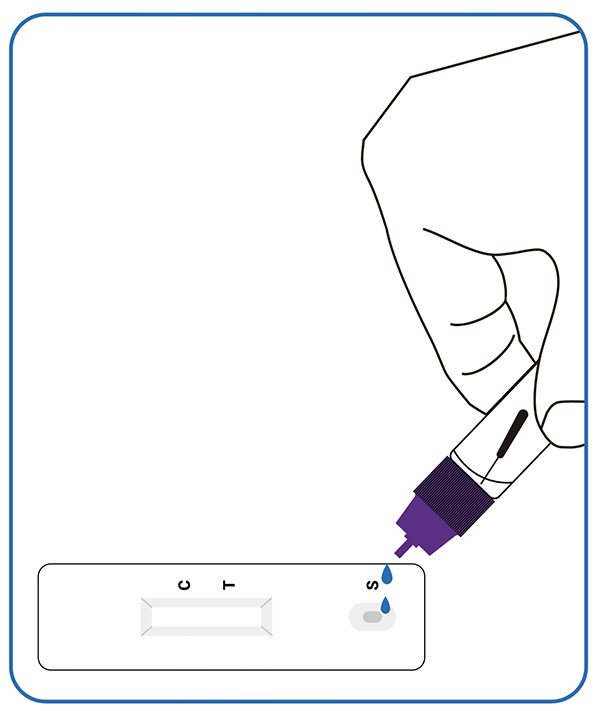
3. Read the result (15-20mins)
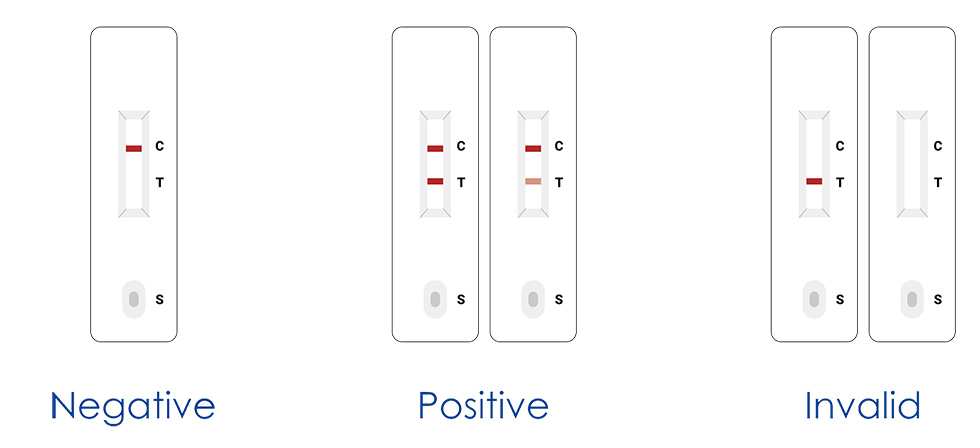
Precautions:
1. Do not read the result after 20 mins.
2. After opening, please use the product within 1 hour.
3. Please add samples in strict accordance with the instructions.
Key Components
| Catalogue Number | Specification | Components | Quantity |
| HWTS-RT062I | 1 test/kit | Test Card | 1 |
| Sample Extraction Solution | 1 | ||
| Disposable Swab | 1 | ||
| Package Insert | 1 | ||
| HWTS-RT062IA | 5 tests/kit | Test Card | 5 |
| Sample Extraction Solution | 5 | ||
| Disposable Swab | 5 | ||
| Package Insert | 1 | ||
| HWTS-RT062IB | 10 tests/kit | Test Card | 10 |
| Sample Extraction Solution | 10 | ||
| Disposable Swab | 10 | ||
| Package Insert | 1 | ||
| HWTS-RT062IC | 20 tests/kit | Test Card | 20 |
| Sample Extraction Solution | 20 | ||
| Disposable Swab | 20 | ||
| Package Insert | 1 |
Reference
[1] New Coronavirus Pneumonia Diagnosis and Treatment Plan. (Eighth Edition), National Health Office Medical Letter [2020] No. 680
Product detail pictures:






Related Product Guide:
We have now probably the most innovative production equipment, experienced and qualified engineers and workers, regarded high quality control systems and also a friendly expert income team pre/after-sales support for Good Quality Covid-19 - SARS-CoV-2 Virus Antigen Detection Kit (colloidal gold)-Home test – Macro & Micro-Test , The product will supply to all over the world, such as: Mali, Albania, British, Our main objectives are to provide our customers worldwide with good quality, competitive price, satisfied delivery and excellent services. Customer satisfaction is our main goal. We welcome you to visit our showroom and office. We are looking forward to establish business relation with you.
The customer service reprersentative explained very detailed, service attitude is very good, reply is very timely and comprehensive, a happy communication! We hope to have a opportunity to cooperate.