Human EGFR Gene 29 Mutations
Product name
HWTS-TM001A-Human EGFR Gene 29 Mutations Detection Kit (Fluorescence PCR)
Epidemiology
Lung cancer has become the leading cause of cancer deaths worldwide, seriously threatening human health. Non-small cell lung cancer accounts for about 80% of lung cancer patients. EGFR is currently the most important molecular target for the treatment of non-small cell lung cancer. The phosphorylation of EGFR can promote tumor cell growth, differentiation, invasion, metastasis, anti-apoptosis, and promote tumor angiogenesis. EGFR tyrosine kinase inhibitors (TKI) can block the EGFR signaling pathway by inhibiting EGFR autophosphorylation, thereby inhibiting the proliferation and differentiation of tumor cells, promoting tumor cell apoptosis, reducing tumor angiogenesis, etc., so as to achieve tumor targeted therapy. A large number of studies have shown that the therapeutic efficacy of EGFR-TKI is closely related to the status of EGFR gene mutation, and can specifically inhibit the growth of tumor cells with EGFR gene mutation. The EGFR gene is located on the short arm of chromosome 7 (7p12), with a full length of 200Kb and consists of 28 exons. The mutated region is mainly located in exons 18 to 21, codons 746 to 753 deletion mutation on exon 19 accounts for about 45% and the L858R mutation on exon 21 accounts for about 40% to 45%. The NCCN Guidelines for the Diagnosis and Treatment of Non-Small Cell Lung Cancer clearly states that EGFR gene mutation testing is required before EGFR-TKI administration. This test kit is used to guide the administration of epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) drugs, and provide the basis for personalized medicine for patients with non-small cell lung cancer. This kit is only used for the detection of common mutations in the EGFR gene in patients with non-small cell lung cancer. The test results are for clinical reference only and should not be used as the sole basis for individualized treatment of patients. Clinicians should consider the patient's condition, drug indications, and treatment The reaction and other laboratory test indicators and other factors are used to comprehensively judge the test results.
Channel
| FAM | IC Reaction Buffer, L858R Reaction Buffer, 19del Reaction Buffer, T790M Reaction Buffer, G719X Reaction Buffer, 3Ins20 Reaction Buffer, L861Q Reaction Buffer, S768I Reaction Buffer |
Technical Parameters
| Storage | Liquid: ≤-18℃ In dark; Lyophilized: ≤30℃ In dark |
| Shelf-life | Liquid: 9 months; Lyophilized: 12 months |
| Specimen Type | fresh tumor tissue, frozen pathological section, paraffin-embedded pathological tissue or section, plasma or serum |
| CV | <5.0% |
| LoD | nucleic acid reaction solution detection under the background of 3ng/μL wild-type, can stably detect 1% mutation rate |
| Specificity | There is no cross-reactivity with wild-type human genomic DNA and other mutant types |
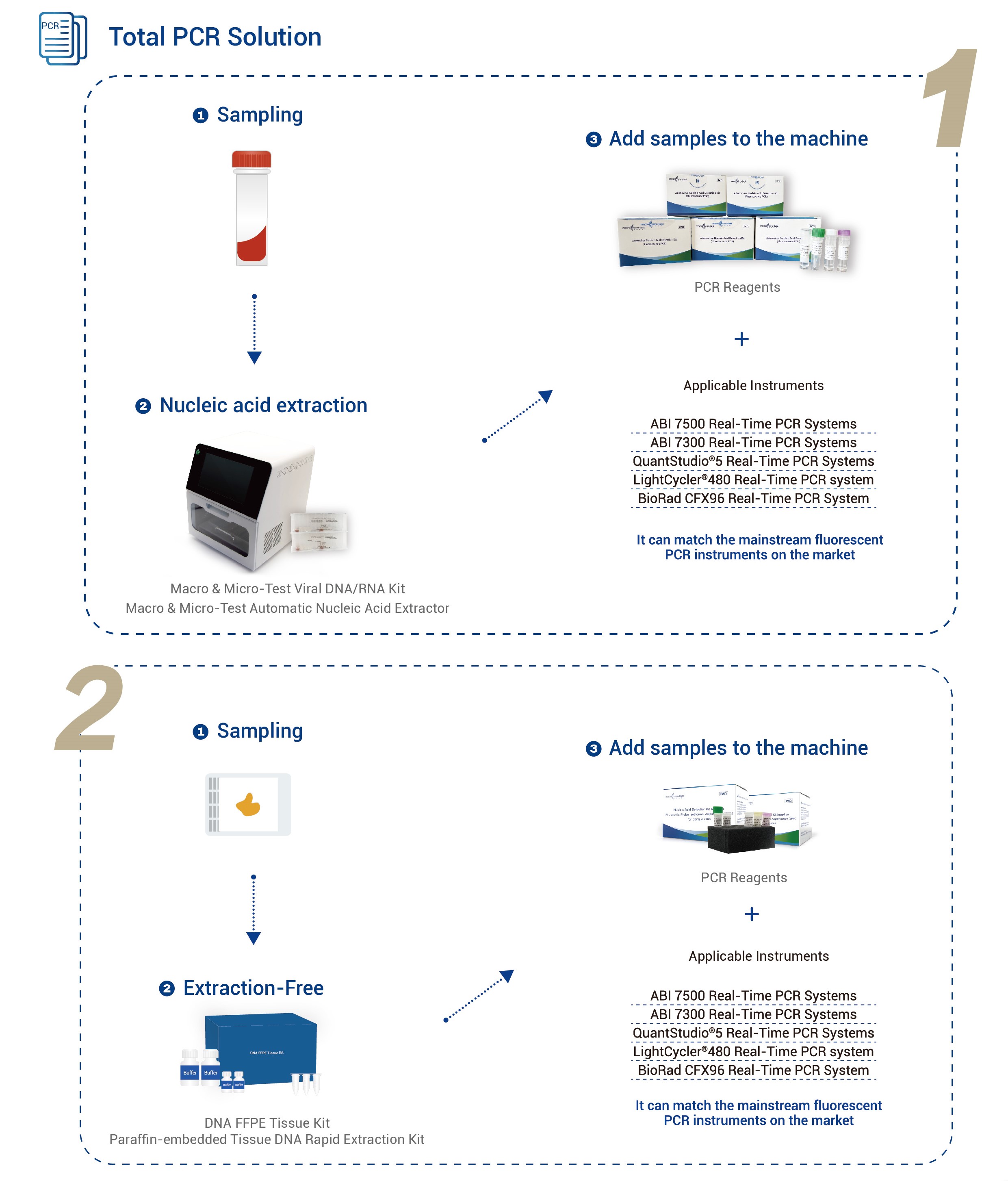
| Applicable Instruments | Applied Biosystems 7500 Real-Time PCR SystemsApplied Biosystems 7300 Real-Time PCR Systems
QuantStudio® 5 Real-Time PCR Systems LightCycler® 480 Real-Time PCR system BioRad CFX96 Real-Time PCR System |