Low MOQ for Hcv Rna - Rapid test molecular platform – Easy Amp – Macro & Micro-Test
Low MOQ for Hcv Rna - Rapid test molecular platform – Easy Amp – Macro & Micro-Test Detail:
Gold standard for nucleic acid detection
Convenient·Portable
Thermostatic inspection system
Molecular platform
Rapid Test
Product name
Easy Amp Real-time Fluorescence Isothermal Detection System
Certificate
CE, FDA, NMPA
Technology platform
Enzymatic Probe Isothermal Amplification
Features
Rapid
Positive sample: within 5 mins.
Visible
Real-time display of detection results.
Easy
4×4 independent heating module design allow on-demand sample detection.
Energy-efficient
Reduced by 2/3 compared with traditional techniques.
Portable
Small size, easy to carry, meet the testing needs in non-laboratory environment.
Accurate
Quantitative detection has a calibration function and outputs quantitative detection results.
Applicable Areas

Airport, Customs, Cruises, Community(Tent), Small Clinics, Mobile Testing Lab, Hospital, etc.
Technical Parameters
| Model | HWTS 1600S | HWTS 1600P |
| Fluorescent Channel | FAM, ROX | FAM, ROX, VIC, CY5 |
| Detection platform | Enzymatic Probe Isothermal Amplification | |
| Capacity | 4 well×200μL×4 groups | |
| Sample volume | 20~60μL | |
| Temperature range | 35~90℃ | |
| Temperature accuracy | ≤±0.5℃ | |
| Excitation light source | High-brightness LED | |
| Printer | Thermal technology instant printing | |
| Semiconductor heating | With rapid speed, stable heat preservation | |
| Storage temperature | -20℃~55℃ | |
| Dimension | 290mm×245mm×128mm | |
| Weight | 3.5KG | |
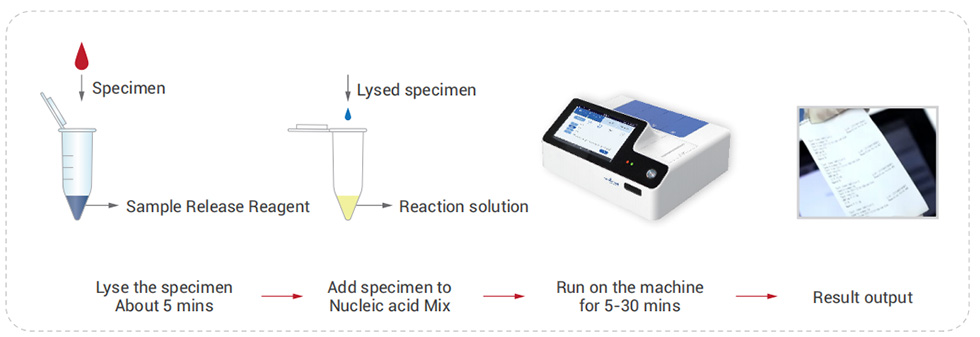
Work Flow
Reagent
| Respiratory tract infection | SARS-CoV-2, Influenza A, Influenza B, Mycobacterium tuberculosis, HRSVa, HRSVb, HRV, HPIV1, HPIV2, HPIV3 |
| Infectious Diseases | Plasmodium, Dengue |
| Reproductive health | Group B Streptococcus, NG, UU, MH, MG |
| Gastrointestinal diseases | Enterovirus, Candida Albicans |
| Else | Zaire, Reston, Sudan |
Easy Amp VS Real-time PCR
| Easy Amp | Real-time PCR | |
| Detection result | Positive sample: within 5 mins | 120 mins |
| Amplification time | 30-60 mins | 120 mins |
| Amplification method | Isothermal amplification | Variable temperature amplification |
| Applicable areas | No special requirements | Only PCR Lab |
| Result output | Thermal technology instant printing | USB copy, printed by printer |
Product detail pictures:





Related Product Guide:
With our abundant experience and considerate products and services, we have been recognized to be a reputable supplier for a lot of global consumers for Low MOQ for Hcv Rna - Rapid test molecular platform – Easy Amp – Macro & Micro-Test , The product will supply to all over the world, such as: Amsterdam, France, Algeria, We adhere to the honest, efficient, practical win-win running mission and people-oriented business philosophy. Excellent quality, reasonable price and customer satisfaction are always pursued! If you are interested in our items, just try to contact us for more details!
The manufacturer gave us a big discount under the premise of ensuring the quality of products, thank you very much, we will select this company again.
Write your message here and send it to us