Low MOQ for Mycobacterium Tuberculosis - Mycobacterium Tuberculosis DNA Detection Kit (Fluorescence PCR) – Macro & Micro-Test
Low MOQ for Mycobacterium Tuberculosis - Mycobacterium Tuberculosis DNA Detection Kit (Fluorescence PCR) – Macro & Micro-Test Detail:
Product name
Mycobacterium Tuberculosis DNA Detection Kit (Fluorescence PCR)
Freeze-dried Mycobacterium Tuberculosis DNA Detection Kit (Fluorescence PCR)
Certificate
CE
Epidemiology
Mycobacterium culosis is referred to as Tubercle bacillus(TB). Mycobacterium tuberculosis that is pathogenic to humans is now generally considered to be of human, bovine, and African types. Its pathogenicity may be related to the inflammation caused by the proliferation of bacteria in tissue cells, the toxicity of bacterial components and metabolites, and the immune damage to the bacterial components. Pathogenic substances are associated with capsules, lipids and proteins.
Mycobacterium tuberculosis can invade susceptible organisms through the respiratory tract, digestive tract or skin injury, causing tuberculosis of various tissues and organs, of which the most common is pulmonary tuberculosis through the respiratory tract. It usually occurs in children, and presents with symptoms such as low-grade fever, night sweats, and a small amount of hemoptysis. Secondary infection is mainly manifested as low-grade fever, night sweats, and hemoptysis. Mostly it is long-term chronic disease. In 2018, about 10 million people around the world were infected with Mycobacterium tuberculosis, of which about 1.6 million died.
Features
● Multiplex PCR Amplification Technology.
● Internal control: Fully monitor the experimental process to ensure the quality of Experiments.
● High sensitivity: 100 bacteria/mL.
● High specificity: No cross-reactivity with the human genome and other non-Mycobacterium tuberculosis and pneumonia pathogens.
● Convenient: One test for multiple results.
Channel
FAM: Target (IS6110 and 38KD) nucleic acid DNA
VIC (HEX): Internal control
PCR Amplification Conditions Setting
| Step | Cycles | Temperature | Time | Collect Fluorescent Signals or Not |
| 1 | 1 cycle | 50℃ | 2mins | No |
| 2 | 1 cycle | 95℃ | 10mins | No |
| 3 | 40 cycles | 95℃ | 15s | No |
| 60℃ | 40s | Yes |
Technical Parameters
| Storage | |
| Liquid | ≤-18℃ In dark |
| Lyophilization | ≤30℃ |
| Shelf-life | 12 months |
| Specimen Type | Sputum |
| Ct | ≤39 |
| CV | ≤5.0% |
| LoD | 100 bacteria /mL |
Specificity: No cross-reactivity with the human genome and other non-Mycobacterium tuberculosis and pneumonia pathogens.
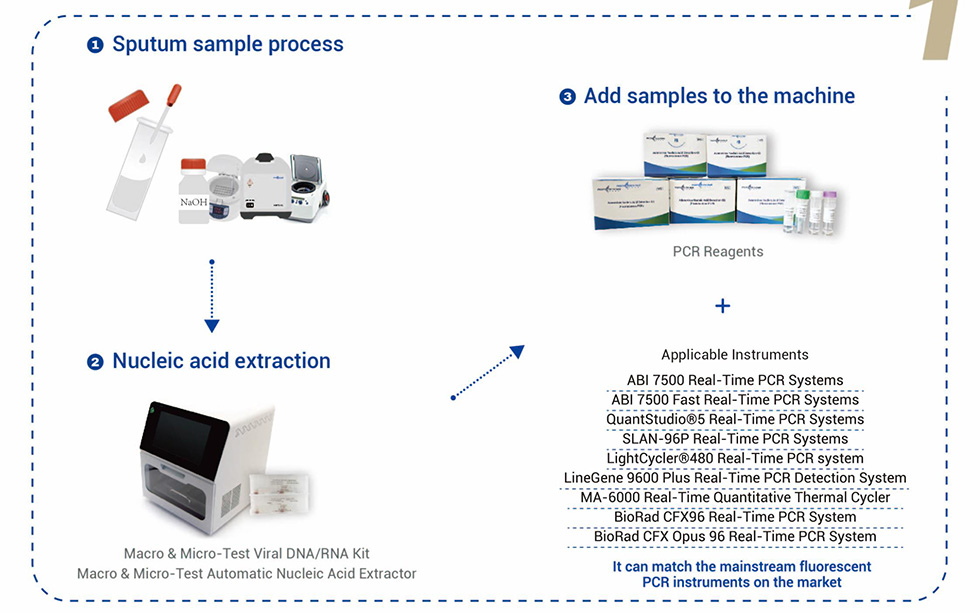
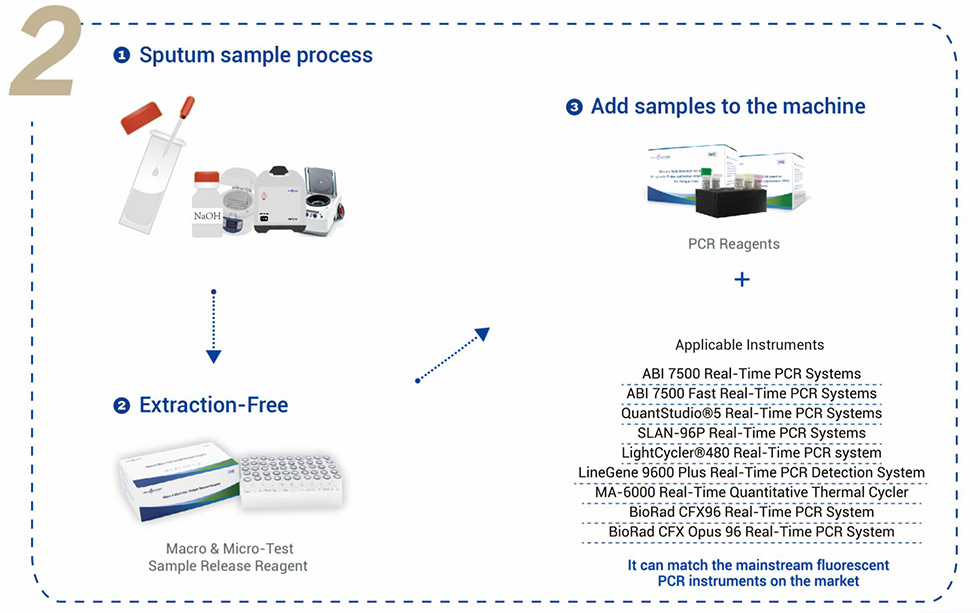
Applicable Instruments: It can match the mainstream fluorescent PCR instruments on the market.
SLAN-96P Real-Time PCR Systems.
ABI 7500 Real-Time PCR Systems.
ABI 7500 Fast Real-Time PCR Systems.
QuantStudio®5 Real-Time PCR Systems.
LightCycler®480 Real-Time PCR Systems.
LineGene 9600 Plus Real-Time PCR Detection Systems.
MA-6000 Real-Time Quantitative Thermal Cycler.
BioRad CFX96 Real-Time PCR System, BioRad.
CFX Opus 96 Real-Time PCR System.
Main Components
Mycobacterium Tuberculosis DNA Detection Kit (Fluorescence PCR)
|
Component |
Catalogue Number |
Quantity |
Component Description |
|
| HWTS-RT001A | HWTS-RT001B | |||
| Specification(50 tests/kit) | Specification(20 tests/kit) | |||
| TB Reaction Buffer | 1 mL/vial | 400μL/vial | 1 vial | Mycobacterium tuberculosis IS6110 and 38KD, internal control specific primers, fluorescent probes, Taq enzyme, UDG enzyme, reaction buffer, etc. |
| TB Positive Control | 1 mL/vial | 400μL/vial | 1 vial | IS6110, 38KD and internal control mixed plasmid diluent |
| TB Blank Control | 1 mL/vial | 400μL/vial | 1 vial | DNase/RNase free H2O |
Freeze-dried Mycobacterium Tuberculosis DNA Detection Kit (Fluorescence PCR)
| Component | Character | Catalogue Number | Component Description | |
| HWTS-RT105A | HWTS-RT105B | |||
| Specification/Quantity(50 tests/kit) | Specification/Quantity(20 tests/kit) | |||
| TB Reaction Buffer | lyophilized | 1 bottle | 1 bottle | Contains Mycobacterium tuberculosis IS6110 and 38KD, internal control specific primers, fluorescent probes, Taq enzyme, UDG enzyme, reaction buffer, etc. |
| TB Positive Control | lyophilized | 1 vial | 1 vial | Contains IS6110, 38KD and internal control mixed plasmid diluent |
| TB Blank Control | Liquid | 1 vial, 600μL/vial | 1 vial, 600μL/vial | DNase/RNase free H2O |
| Reconstituted Solution | Liquid | 1 vial, 1.45mL/vial | 1 vial, 1mL/vial | DNase/RNase free H2O, etc. |
Total PCR Solution
Option 1.
Option 2.
Reference
[1]. Programme W. Global tuberculosis control: WHO report [annual].[J]. Who Global Tuberculosis Programme, 2003.
[2]. Kocagoz T, Yilmaz E, Ozkara S, et al. Detection of Mycobacterium tuberculosis in sputum samples by polymerase chain reaction using a simplified procedure.[J]. Journal of clinical microbiology, 1993, 31(6):1435-1438.
[3]. Mikhailovich V, Lapa S, Gryadunov D, et al. Identification of Rifampin-Resistant Mycobacterium tuberculosis Strains by Hybridization, PCR, and Ligase Detection Reaction on Oligonucleotide Microchips[J]. Journal of Clinical Microbiology, 2001, 39(7):2531-2540.
[4]. Miller M B, Popowitch E B, Backlund M G, et al. Performance of Xpert MTB/RIF RUO Assay and IS6110 Real-Time PCR for Mycobacterium tuberculosis Detection in Clinical Samples[J]. Journal of Clinical Microbiology, 2011, 49(10):3458-62.
Product detail pictures:







Related Product Guide:
Assume full responsibility to meet all demands of our clients; achieve continuous advancements by promoting the growth of our clients; become the final permanent cooperative partner of clients and maximize the interests of clients for Low MOQ for Mycobacterium Tuberculosis - Mycobacterium Tuberculosis DNA Detection Kit (Fluorescence PCR) – Macro & Micro-Test , The product will supply to all over the world, such as: Sao Paulo, UAE, Jakarta, We take measure at any expense to achieve essentially the most up-to-date equipment and approaches. The packing of nominated brand is our a further distinguishing feature. The items to assure years of trouble-free service has attracted a great deal customers. The solutions are obtainable in improved designs and richer assortment, they're created scientifically of purely raw supplies. It readily available in a variety of designs and specifications for your selection. The most recent kinds are a great deal better than the preceding one particular and they are quite popular with lots of prospects.
The product manager is a very hot and professional person, we have a pleasant conversation, and finally we reached a consensus agreement.