Monkeypox Virus Nucleic Acid Detection Kit (Fluorescence PCR) – Macro & Micro-Test
Monkeypox Virus Nucleic Acid Detection Kit (Fluorescence PCR) – Macro & Micro-Test Detail:
Product name
Monkeypox Virus Nucleic Acid Detection Kit (Fluorescence PCR)
Orthopox Virus Universal Type/Monkeypox Virus Nucleic Acid Detection Kit (Fluorescence PCR)
Certificate
CE
Epidemiology
Monkeypox (MP) is an acute zoonotic infectious disease caused by Monkeypox Virus (MPV). The disease is mainly transmitted by animals, and humans could become infected by being bitten by infected animals or by direct contact with the blood, body fluids and rash of infected animals. The virus can also be transmitted between people, primarily through respiratory droplets during prolonged, direct face-to-face contact or through direct contact with a patient’s body fluids or contaminated objects[1-2].
The clinical symptoms of monkeypox infection in humans are similar to those of smallpox, generally after a 12-day incubation period, appearing fever, headache, muscle and back pain, enlarged lymph nodes, fatigue and discomfort. The rash appears after 1-3 days of the fever, usually first on the face, but also in other parts. The disease course generally lasts 2-4 weeks, and the mortality rate is 1%-10%. Lymphadenopathy is one of the main differences between this disease and smallpox[3].
Features
● Multiplex PCR Amplification Technology
● Accuracy: Internal control, fully monitor the experimental process to ensure the quality of Experiments.
● Rapid: Rapid PCR amplification shorts detection time, resulted in 40 minutes
● High sensitivity: 200 Copies/mL
● Triple PCR assay: Dual target gene(MPV-1 & MPV-2) of Monkeypox virus + human endogenous gene
Channel
| Channel | Monkeypox | Monkeypox & Orthopox |
| FAM | Monkeypox virus MPV-1 gene | Orthopox virus universal type nucleic acid |
| VIC/HEX | Monkeypox virus MPV-2 gene | Monkeypox virus MPV-2 gene |
| ROX | / | Monkeypox virus MPV-1 gene |
| CY5 | Internal Control | Internal control |
PCR Amplification Conditions Setting
| Step | Cycles | Temperature | Time | Collect Fluorescent Signals or Not |
| 1 | 1 cycle | 50℃ | 2mins | No |
| 2 | 1 cycle | 95℃ | 1min | No |
| 3 | 40 cycles | 95℃ | 5s | No |
| 55℃ | 15s | Yes |
Technical Parameters
| Storage | ≤-18℃ in dark |
| Shelf-life | 2 months |
| Specimen Type | Rash Fluid, Nasopharyngeal Swab, Throat Swab, Serum |
| Ct | ≤38 |
| CV | ≤5.0% |
| LoD | 200Copies/mL |
| Specificity | |
| No cross-reactivity with Smallpox virus, Cowpox virus, Vaccinia virus, Herpes simplex virus, etc. | |
| No cross-reactivity with other pathogens that cause rash disease. | |
| No cross-reactivity with human genomic DNA. | |
| Applicable Instruments | It can match the mainstream fluorescent PCR instruments on the market. |
| QuantStudio®5 Real-Time PCR Systems | |
| SLAN-96P Real-Time PCR Systems | |
| LightCycler®480 Real-Time PCR Systems | |
| LineGene 9600 Plus Real-Time PCR Detection Systems | |
| MA-6000 Real-Time Quantitative Thermal Cycler | |
| BioRad CFX96 Real-Time PCR Systems | |
| BioRad CFX Opus 96 Real-Time PCR Systems | |
Main Components
Monkeypox Virus Nucleic Acid Detection Kit (Fluorescence PCR)
| Catalogue Number | Component (50 tests/kit) | Specification | Quantity | Component Description |
| HWTS-OT071A | MPV Reaction Buffer | 1.25mL /vial | 1 vial | Amplification reaction reagents, monkeypox virus MPV-1 and MPV-2 primer probes and internal control primer probes, etc.. |
| MPV Positive Control | 250μL/vial | 1 vial | Monkeypox virus and internal control mixed plasmid | |
| MPV Blank Control | 250μL/vial | 1 vial | DNase/RNase free H2O |
Orthopox Virus Universal Type/Monkeypox Virus Nucleic Acid Detection Kit (Fluorescence PCR)
| Catalogue Number | Component (50 tests/kit) | Specification | Quantity | Component Description |
| HWTS-OT072A | OPV/MPV Reaction Buffer | 1.25mL /vial | 1 vial | Amplification reaction reagents, orthopox virus universal type and monkeypox virus primer probes, internal control primer probes, etc. |
| OPV/MPV Positive Control | 250μL/vial | 1 vial | Orthopox virus universal type, monkeypox virus and internal control mixed plasmid | |
| OPV/MPV Blank Control | 250μL/vial | 1 vial | DNase/RNase free H2O |
Total PCR Solution
Option 1.
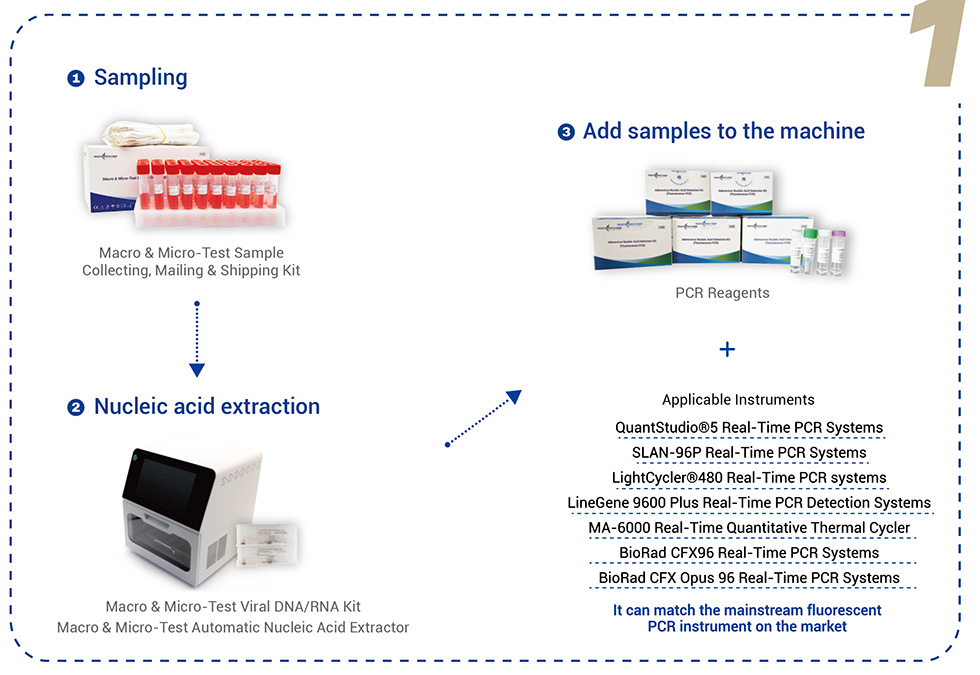
Option 2.

References
[1] Centers for Disease Control and Prevention. Human monkeypox-Kasai Oriental, Zaire, 1996-1997.MMWR. 1997, 46:304-307.
[2] Beer EM, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis 2019 10;13(10).
[3] Jezek Z, Marennikova SS, Mutumbo M, et al. Human monkeypox: a study of 2,510 contacts of 214 patients. J Infect Dis 1986 Oct;154(4).
Product detail pictures:



Related Product Guide:
We insist on the principle of development of 'High quality, Efficiency, Sincerity and Down-to-earth working approach' to deliver you with great provider of processing for Monkeypox Virus Nucleic Acid Detection Kit (Fluorescence PCR) – Macro & Micro-Test , The product will supply to all over the world, such as: Iraq, Mexico, Accra, Our solutions are produced with the best raw materials. Every moment, we constantly improve the production programme. In order to ensure better quality and service, we now have been focusing on the production process. We have got high praise by partner. We are looking forward to establishing business relationship with you.
The goods are very perfect and the company sales manager is warmful, we will come to this company to purchase next time.