New Delivery for Mycobacterium Test - Mycobacterium Tuberculosis DNA Detection Kit (Isothermal Amplification) – Macro & Micro-Test
New Delivery for Mycobacterium Test - Mycobacterium Tuberculosis DNA Detection Kit (Isothermal Amplification) – Macro & Micro-Test Detail:
Product name
Adenovirus Type 41 Nucleic Acid Detection Kit(Fluorescence PCR)
Certificate
CE
Packaging Size
1. Liquid product: 50 tests/kit
2. Freeze-dried product (penicillin vial): 20 tests/kit, 50 tests/kit
3. Freeze-dried product (8-tube strips): 48 tests/kit
Intended Use
This kit is used for the qualitative detection of adenovirus nucleic acid in stool samples in vitro.
Epidemiology
Adenovirus (Adv) belongs to the Adenovirus family. Adv can proliferate and cause disease in cells of the respiratory tract, gastrointestinal tract, urethra, and conjunctiva. It is mainly infected through the gastrointestinal tract, respiratory tract or close contact, especially in swimming pools with insufficient disinfection, which can increase the chance of transmission and cause outbreaks[1-2].
Adv mainly infects children. The gastrointestinal tract infections in children are mainly type 40 and 41 in group F. Most of them have no clinical symptoms, and some cause diarrhea in children. Its mechanism of action is to invade the small intestinal mucosa of children, making the intestinal mucosal epithelial cells smaller and shorter, and the cells degenerate and dissolve, resulting in intestinal absorption dysfunction and diarrhea. Abdominal pain and bloating may also occur, and in severe cases, the respiratory system, central nervous system, and extraintestinal organs such as liver, kidney, and pancreas may be involved and the disease may be aggravated[3-5].
Features
Multiplex PCR Amplification Technology
Internal control: Fully monitor the experimental process to ensure the quality of Experiments.
High sensitivity: 300Copies/mL
High specificity: No cross-reactivity with common respiratory pathogens or bacteria for more accurate results.
Rapid: Rapid PCR amplification, test results can be obtained in 40 minutes.
Channel
FAM: Adenovirus type 41 nucleic acid
VIC (HEX): Internal control
Amplification Conditions Setting
| Step | Cycles | Temperature | Time | Collect Fluorescent Signals or Not |
| 1 | 1 cycle | 50℃ | 2mins | No |
| 2 | 1 cycle | 95℃ | 1min | No |
| 3 | 40 cycles | 95℃ | 5s | No |
| 55℃ | 15s | Yes |
Technical Parameters
Storage:
Liquid: ≤-18℃ In dark
Lyophilization: ≤30℃
Shelf-life: 12 months
Specimen Type: Stool samples
Ct: ≤38
CV: ≤5.0%
LoD: 300Copies/mL
Specificity: Use the kits to detect other respiratory pathogens (such as influenza A virus, influenza B virus, respiratory syncytial virus, parainfluenza virus, rhinovirus, human metapneumovirus, etc.) or bacteria (streptococcus pneumoniae, klebsiella pneumoniae, pseudomonas aeruginosa, acinetobacter baumannii, staphylococcus aureus, etc.) and common gastrointestinal pathogens group A rotavirus, escherichia coli, etc. There is no cross-reactivity with all the pathogens or bacteria mentioned above.
Applicable Instruments: It can match the mainstream fluorescent PCR instruments on the market.
ABI 7500 Real-Time PCR Systems
ABI 7500 Fast Real-Time PCR Systems
SLAN-96P Real-Time PCR Systems
QuantStudio®5 Real-Time PCR Systems
LightCycler®480 Real-Time PCR Systems
LineGene 9600 Plus Real-Time PCR Detection Systems
MA-6000 Real-Time Quantitative Thermal Cycler
Main Components
Liquid product
|
Catalogue Number |
Component (50 tests/kit) |
Specification |
Quantity |
Component Description |
|
HWTS-RT113B |
ADV 41 Reaction Buffer
|
1.25mL /vial |
1 vial |
Amplification reaction reagents, adenovirus type 41 primer probes, internal control primer probes, etc. |
|
ADV 41 Positive Control |
250μL/vial
|
1 vial |
Adenovirus and internal control plasmids mixture. |
|
|
ADV 41 Blank Control |
250μL/vial |
1 vial |
DNase/RNase free H2O |
Lyophilized product
| Component | Character | Catalogue Number | Component Description | ||
| HWTS-RT113A | HWTS-RT113C | HWTS-RT113Z | |||
| Specification/Quantity(20 tests/kit) | Specification/Quantity(50 tests/kit) | Specification/Quantity(48 tests/kit) | |||
| ADV 41 Reaction Buffer | lyophilized | 1 bottle | 1 bottle | 8-tube strips, 6 strips | Amplification reaction reagents, adenovirus type 41 primer probes, internal control primer probes, etc. |
| ADV 41 Positive Control | lyophilized | 1 vial | 1 vial | 1 vial | Adenovirus and internal control plasmids mixture. |
| ADV 41 Blank Control | Liquid | 1 vial, 250μL/vial | 1 vial, 250μL/vial | 1 vial, 250μL/vial | DNase/RNase free H2O |
| ADV 41 Reconstituted Solution | Liquid | 1 vial, 650μL/vial | 1 vial, 1.25mL/vial | 1 vial, 1.21mL/vial | DNase/RNase free H2O, etc. |
Total PCR Solution
Option 1.
[References]
[1] Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study[J]. Lancet, 2016, 388(10051):1291-1301.
[2] Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study.[J]. Lancet, 2013, 382(9888):209-222.
[3] Liu M, Worley S, Arrigain S, et al. Respiratory viral infections within one year after pediatric lung transplant[J]. Transplant Infectious Disease, 2010, 11(4):304-312.
[4] Dey R S, Ghosh S, Chawla-Sarkar M, et al. Circulation of a Novel Pattern of Infections by Enteric Adenovirus Serotype 41 among Children below 5 Years of Age in Kolkata, India[J]. Journal of Clinical Microbiology, 2011, 49(2):500-505.
[5] Lekana-Douki S E, Kombila-Koumavor C, Nkoghe D, et al. Molecular epidemiology of enteric viruses and genotyping of rotavirus A, adenovirus and astrovirus among children under 5 years old in Gabon[J]. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases, 2015, 34(C):90-95.




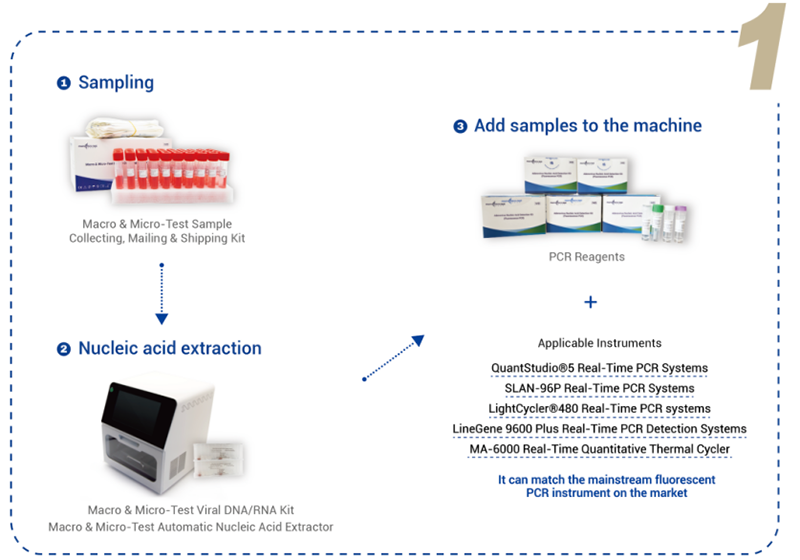
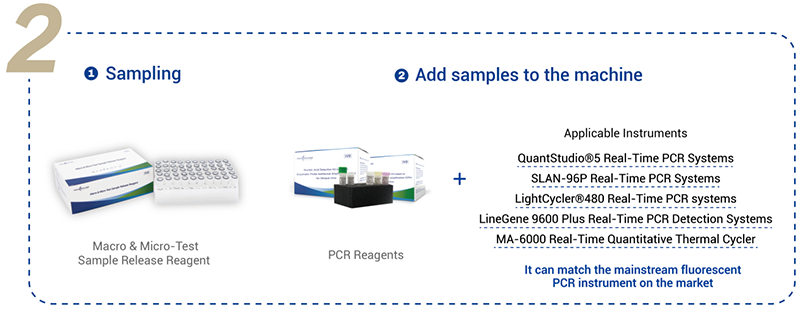
Product detail pictures:





Related Product Guide:
We keep on with our business spirit of Quality, Performance, Innovation and Integrity. We goal to create much more worth for our customers with our rich resources, state-of-the-art machinery, experienced workers and exceptional providers for New Delivery for Mycobacterium Test - Mycobacterium Tuberculosis DNA Detection Kit (Isothermal Amplification) – Macro & Micro-Test , The product will supply to all over the world, such as: Tunisia, United Kingdom, Jeddah, Based on products with high quality, competitive price, and our full range service, we have accumulated professional strength and experience, and we have built up a very good reputation in the field. Along with the continuous development, we commit ourselves not only to the Chinese domestic business but also the international market. May you moved by our high quality products and passionate service. Let's open a new chapter of mutual benefit and double win.
We have been engaged in this industry for many years, we appreciate the work attitude and production capacity of the company, this is a reputable and professional manufacturer.