01 What is GBS?
Group B Streptococcus (GBS) is a Gram-positive streptococcus that resides in the lower digestive tract and genitourinary tract of the human body. It is an opportunistic pathogen.GBS mainly infects the uterus and fetal membranes through the ascending vagina. GBS can cause maternal urinary tract infection, intrauterine infection, bacteremia and postpartum endometritis, and increase the risk of premature delivery or stillbirth.
GBS can also lead to neonatal or infant infection. About 10%-30% of pregnant women suffer from GBS infection. 50% of these can be transmitted vertically to the newborn during delivery without intervention, resulting in neonatal infection.
According to the onset time of GBS infection, it can be divided into two types, one is GBS early-onset disease (GBS-EOD) ,which occurs 7 days after delivery, mainly occurs 12-48 hours after delivery, and mainly manifests as neonatal Bacteremia, pneumonia, or meningitis. The other is GBS late-onset disease (GBS-LOD), which occurs from 7 days to 3 months postpartum and manifests mainly as neonatal/infant bacteremia, meningitis, pneumonia, or organ and soft tissue infection.
Prenatal GBS screening and intrapartum antibiotic intervention can effectively reduce the number of neonatal early-onset infections, increase neonatal survival rate and quality of life.
02 How to prevent?
In 2010, the US Centers for Disease Control and Prevention (CDC) formulated the "Guidelines for the Prevention of Perinatal GBS", recommending routine screening for GBS at 35-37 weeks of pregnancy in the third trimester.
In 2020, the American College of Obstetricians and Gynecologists (ACOG) "Consensus on the Prevention of Early-onset Group B Streptococcal Disease in Newborns" recommends that all pregnant women should undergo GBS screening between 36+0-37+6 weeks of pregnancy.
In 2021, the "Expert Consensus on the Prevention of Perinatal Group B Streptococcal Disease (China)" issued by the Perinatal Medicine Branch of the Chinese Medical Association recommends GBS screening for all pregnant women at 35-37 weeks of gestation. It recommends that the GBS screening is valid for 5 weeks. And if the GBS negative person has not delivered for more than 5 weeks, it is recommended to repeat the screening.
03 Solution
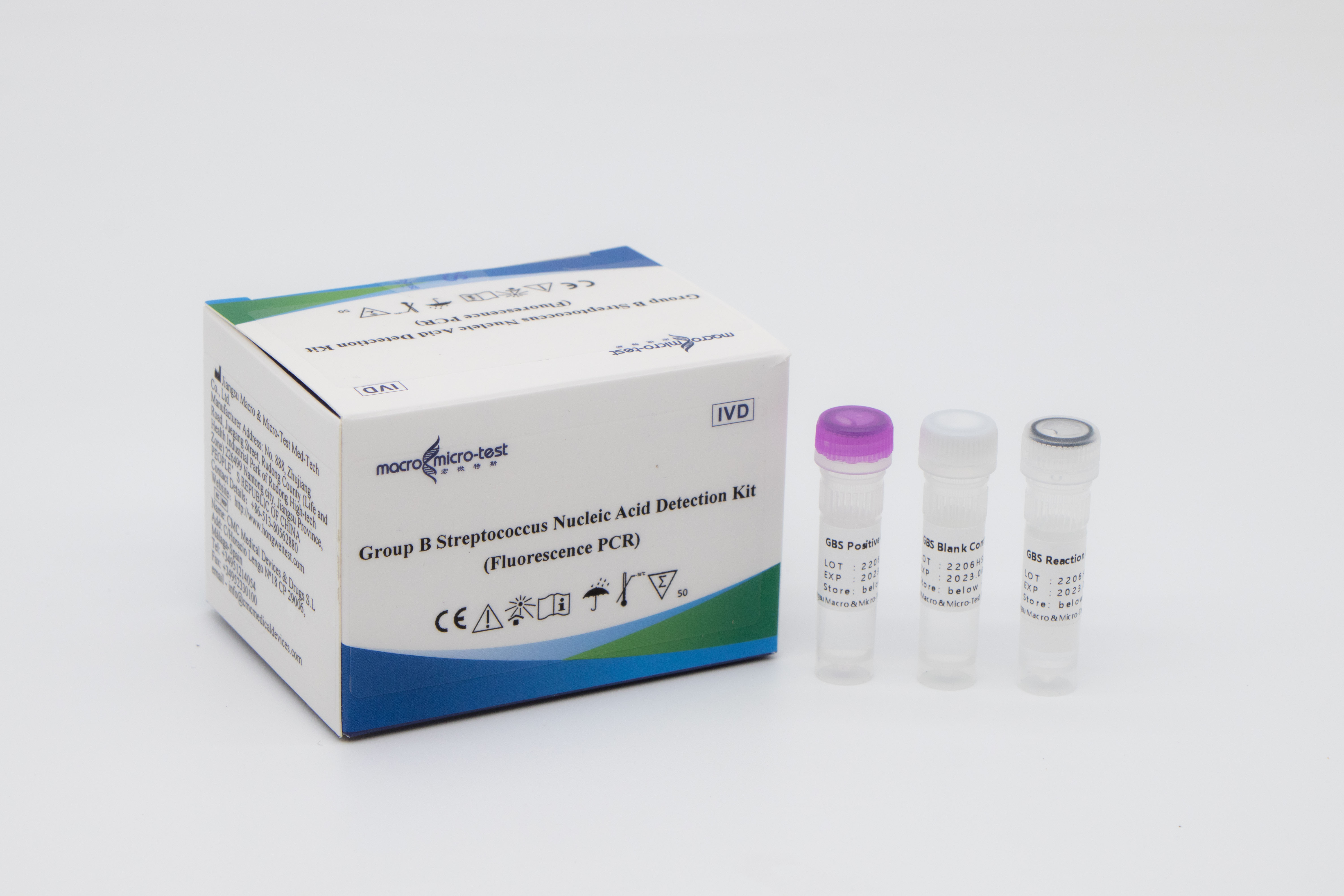
Macro & Micro-Test has developed Group B Streptococcus Nucleic Acid Detection Kit(Fluorescence PCR), which detects samples such as human reproductive tract and rectal secretions to evaluate the status of group B streptococcal infection, and assist pregnant women with GBS infection diagnosis. The product has been certified by EU CE and US FDA, and has excellent product performance and good user experience.
 |
 |
Advantages
Rapid: Simple sampling, one-step extraction, rapid detection
High sensitivity: the LoD of the kit is 1000 Copies/mL
Multi-subtype: including 12 subtypes such as la, lb, lc, II, III
Anti-pollution: UNG enzyme is added to the system to effectively prevent nucleic acid pollution in the laboratory
| Catalogue Number | Product Name | Specification |
| HWTS-UR027A | Group B Streptococcus Nucleic Acid Detection Kit(Fluorescence PCR) | 50 tests/kit |
| HWTS-UR028A/B | Freeze-dried Group B Streptococcus Nucleic Acid Detection Kit(Fluorescence PCR) | 20 tests/kit50 tests/kit |
Post time: Dec-15-2022
