Tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB), remains a global health threat, and the increasing resistance to key TB drugs like Rifampicinn (RIF) and Isoniazid (INH) is critical as the obstacle to global TB control efforts. Rapid and accurate molecular test of TB and resistance to RIF& INH is recommended by the WHO to identify the infected patients timely and provide them with appropriate in-time treatment.
Challenges
An estimated 10.6 million people fell ill with TB in 2022, resulting in an estimated 1.3 million deaths, far from the 2025 milestone of the End TB Strategy
Drug-resistant TB, particularly MDR-TB (resistant to RIF & INH), is increasingly affecting the global TB treatment and prevention.
Rapid simultaneous TB and RIF/INH resistance diagnosis urgently required for earlier and more effective treatment compared with delayed drug susceptibility testing results.
Our Solution
Marco & Micro-Test’s 3-in-1 TB Detection for TB infection/RIF & NIH Resistance Detection Kit enables efficient diagnosis of TB and RIF/INH in one detection.
Melting curve technology realizes simultaneous detection of TB and MDR-TB.
3-in-1 TB/MDR-TB detection determining TB infection and key first-line drugs (RIF/INH) resistance enables timely and accurate TB treatment.
Successfully realizes the triple TB testing (TB infection, RIF & NIH Resistance) in one detection!
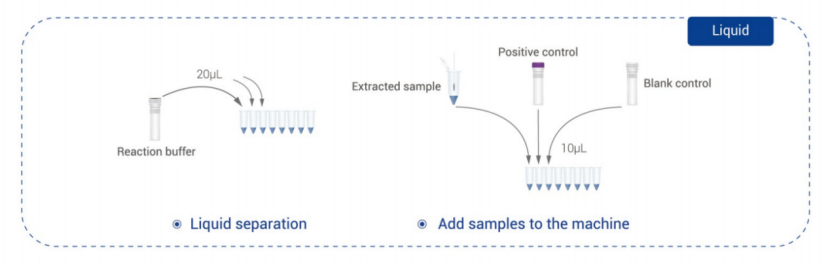
Rapid result: Available in 2-2.5 hrs with automatic result interpretation minimizing technical training for operation;
Test Sample: sputum, solid culture, liquid culture
High Sensitivity: 25 bacteria/mL for TB, 200 bacteria/mL for RIF resistant bacteria, 400 bacteria/mL for INH resistant bacteria, ensuring reliable detection even at low bacterial loads.
Multiple Targets: TB-IS6110; RIF-resistance-rpoB (507~503); INH-resistance-InhA, AhpC, katG 315;
Quality Validation: Internal control for sample quality validation to reduce false negatives;
Wide Compatibility: Compatibility with most mainstream PCR systems for wide lab accessibility (Bio-Rad CFX96, SLAN-96P/96S, Bioer Quantgene 9600);
WHO Guidelines Compliance: Adhering to WHO guidelines for the management of drug-resistant tuberculosis, ensuring reliability and relevance in clinical practice.
Post time: Jul-08-2024
