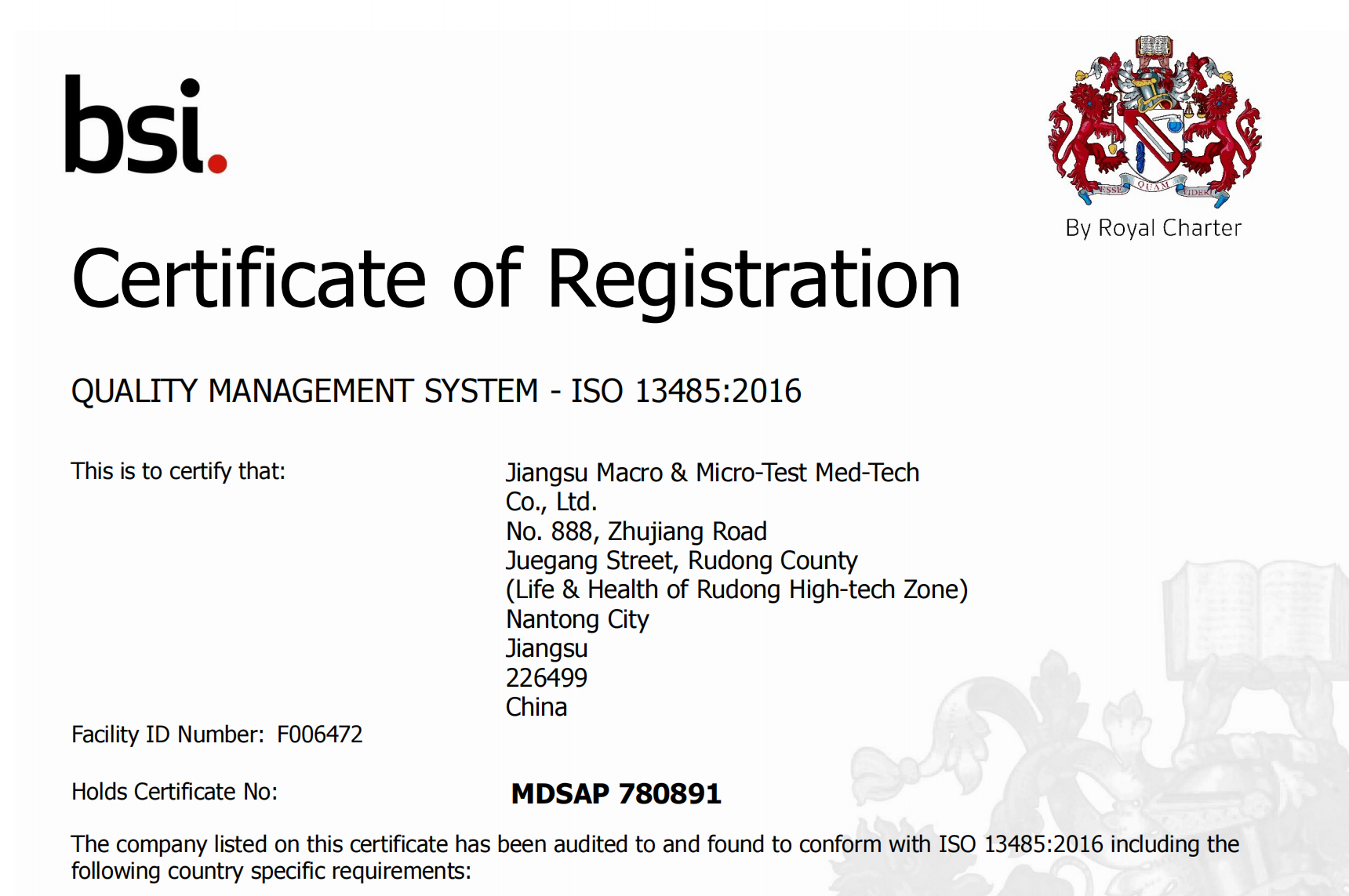
We are delighted to announce receipt of Medical Device Single Audit Program certification (#MDSAP). MDSAP will support commercial approvals for our products in the five countries, including Australia, Brazil, Canada, Japan and the US.
MDSAP allows the conduct of a single regulatory audit of a medical device manufacturer’s quality management system to satisfy the requirements of multiple regulatory jurisdictions or authorities enabling appropriate regulatory oversight of medical device manufacturers’ quality management systems while minimizing regulatory burden on the industry. The program currently represents Australia’s Therapeutic Goods Administration, Brazil’s Agência Nacional de Vigilância Sanitária, Health Canada, Japan’s Ministry of Health, Labour and Welfare and Pharmaceutical and Medical Devices Agency, and the U.S. Food and Drug Administration’s Center for Devices and Radiological Health.
Post time: Apr-13-2023