OEM Manufacturer Hpv Test - 18 Types of High-risk Human Papilloma Virus Nucleic Acid Detection Kit – Macro & Micro-Test
OEM Manufacturer Hpv Test - 18 Types of High-risk Human Papilloma Virus Nucleic Acid Detection Kit – Macro & Micro-Test Detail:
Product name
18 Types of High-risk Human Papilloma Virus Nucleic Acid Detection Kit (Fluorescence PCR)
Certificate
CE
Epidemiology
Cervical cancer is one of the most common malignant tumors in the female reproductive tract. Studies have shown that persistent infection and multiple infections of human papillomavirus are one of the important causes of cervical cancer.
Reproductive tract HPV infection is common among women with sexual life. According to statistics, 70% to 80% of women may have HPV infection for once at least in their lifetime, but most infections are self-limiting, and more than 90% of infected women will develop an effective immune response that could clear the infection between 6 and 24 months without any long-term health intervention. Persistent high-risk HPV infection is the main cause of cervical intraepithelial neoplasia and cervical cancer.
The worldwide study results showed that the presences of high-risk HPV DNA were detected in 99.7% of cervical cancer patients. Therefore, the early detection and prevention of cervical HPV are the key to blocking canceration. The establishment of a simple, specific and rapid pathogenic diagnostic method is of great significance in the clinical diagnosis of cervical cancer.
Features
● Multiplex PCR Amplification Technology.
● Internal control: Fully monitor the experimental process to ensure the quality of Experiments.
● High specificity: No cross-reactivity with common respiratory pathogens for more accurate results.
● High sensitivity: 25 copies/reaction.
● Convenient: One test for multiple results.
Channel
| FAM | HPV 18 |
| VIC (HEX) | HPV 16 |
| ROX | HPV 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82 |
| CY5 | Internal control |
PCR Amplification Conditions Setting
| Step | Cycles | Temperature | Time | Collect Fluorescent Signals or Not |
| 1 | 1 cycle | 50℃ | 2 mins | No |
| 2 | 1 cycle | 95℃ | 10 mins | No |
| 3 | 10 cycle | 95℃ | 15 s | No |
| 62℃ | 20 s | No | ||
| 4 | 30 cycle | 95℃ | 15 s | No |
| 58℃ | 30 s | Yes |
Technical Parameters
| Storage | ≤-18℃ in dark |
| Shelf-life | 24 months |
| Specimen Type | Cervical exfoliated cells |
| Ct | ≤28 |
| CV | ≤5.0% |
| LoD | 25 Copies/reaction |
| Specificity | No cross-reactivity with common reproductive tract pathogens (such as ureaplasma urealyticum, genital tract chlamydia trachomatis, candida albicans, neisseria gonorrhoeae, trichomonas vaginalis, mold, gardnerella and other HPV types not covered in the kit, etc). |
| Applicable Instruments | It can match the mainstream fluorescent PCR instruments on the market. |
| SLAN-96P Real-Time PCR Systems | |
| ABI 7500 Real-Time PCR Systems | |
| QuantStudio®5 Real-Time PCR Systems | |
| LightCycler®480 Real-Time PCR Systems | |
| LineGene 9600 Plus Real-Time PCR Detection Systems | |
| MA-6000 Real-Time Quantitative Thermal Cycler | |
Main Components
| Catalogue Number | Component (50 tests/kit) | Specification | Quantity | Component Description |
| HWTS-CC011A | 18 HPV Reaction Buffer | 1.25mL/vial | 1 vial | Amplification reaction reagents and kinds of primers, probes |
| 18 HPV Positive Control | 600μL/vial | 1 vial | Mixture of HPV 16, 18, 33 and their internal control plasmids | |
| 18 HPV Blank Control | 600μL/vial | 1 vial | DNase/RNase free H2O |
Total PCR Solution
Option 1.
1. Sampling
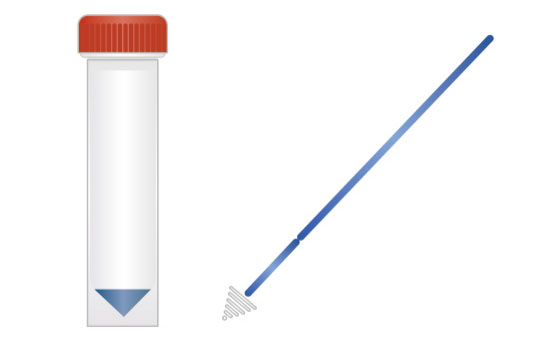
2. Nucleic acid extraction

3. Add samples to the machine

Option 2.
1. Sampling

2. Extraction-free

3. Add samples to the machine

Product detail pictures:
Related Product Guide:
abide by the contract, conforms on the market requirement, joins from the market competition by its good quality likewise as provides more comprehensive and superb support for customers to let them become large winner. The pursue of the company, is definitely the clients' pleasure for OEM Manufacturer Hpv Test - 18 Types of High-risk Human Papilloma Virus Nucleic Acid Detection Kit – Macro & Micro-Test , The product will supply to all over the world, such as: Rio de Janeiro, Swiss, Melbourne, Profession, Devoting are always fundamental to our mission. We've got always been in line with serving customers, creating value management objectives and adhering to the sincerity, dedication, persistent management idea.
This is the first business after our company establish, products and services are very satisfying, we have a good start, we hope to cooperate continuous in the future!