Personlized Products Group A Rotavirus - Macro & Micro-Test Sample Release Reagent – Macro & Micro-Test
Personlized Products Group A Rotavirus - Macro & Micro-Test Sample Release Reagent – Macro & Micro-Test Detail:
Product name
Macro & Micro-Test Sample Release Reagent
Certificate
CE, FDA, NMPA
Features
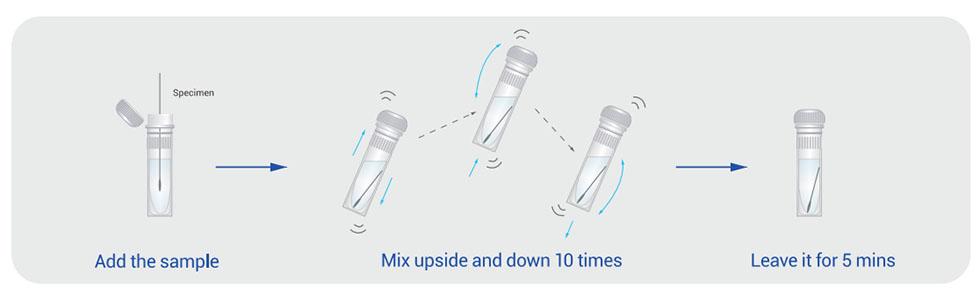
Rapid: only 5 mins.
Easy: only 3 steps (1.add the sample 2.Mix upside and down 10 times 3. Leave it for 5 mins).
Main components
| Name | Main components | Component specifications | Quantity |
| Sample Release reagent | Dithiothreitol, sodium dodecyl sulfate (SDS), RNase inhibitor, surfactant, purified water | 0.5mL/Vial | 50 Vial |
Note: Components in different batches of kits are not interchangeable.
Storage conditions and shelf life
Store and transport at room temperature. The shelf life is 24 months.
Applicable instruments
Instruments and equipment during sample processing, such as pipettes, vortex mixers, water baths, etc.
Sample requirements
Freshly collected oropharyngeal swabs, nasopharyngeal swabs.
Precision
When this kit is used for extraction from the in-house precision reference CV for 10 replicates, the coefficient of variation (CV, %) of Ct value is not more than 10%.
Inter-batch difference
When the in-house precision reference is tested on three batches of kits under trial production upon repeated extraction and, the coefficient of variation (CV, %) of Ct value is not more than 10%.
Performance comparison
● Extraction efficiency comparsion
|
Efficiency comparsion of magnetic beads method and sample releaser |
||||
|
concentration |
magnetic beads method |
sample releaser |
||
|
orfab |
N |
orfab |
N |
|
|
20000 |
28.01 |
28.76 |
28.6 |
29.15 |
|
2000 |
31.53 |
31.9 |
32.35 |
32.37 |
|
500 |
33.8 |
34 |
35.25 |
35.9 |
|
200 |
35.25 |
35.9 |
35.83 |
35.96 |
|
100 |
36.99 |
37.7 |
38.13 |
undet |
The extraction efficiency of sample releaser was similar to that of magnetic beads method, and the concentration of pathogen could be 200Copies/mL.
● CV value comparison
|
Repeatability of sample releaser extraction |
||
| concentration:5000Copies/mL |
ORF1ab |
N |
|
30.17 |
30.38 |
|
|
30.09 |
30.36 |
|
|
30.36 |
30.26 |
|
|
30.03 |
30.48 |
|
|
30.14 |
30.45 |
|
|
30.31 |
30.16 |
|
|
30.38 |
30.7 |
|
|
30.72 |
30.79 |
|
| CV |
0.73% |
0.69% |
When tested at 5,000 copies /mL, the CV of orFab and N was 0.73% and 0.69%, respectively.
Product detail pictures:


Related Product Guide:
Bear Customer 1st, Good quality first in mind, we work closely with our prospects and supply them with efficient and professional services for Personlized Products Group A Rotavirus - Macro & Micro-Test Sample Release Reagent – Macro & Micro-Test , The product will supply to all over the world, such as: Johannesburg, Dominica, Swiss, Our company has built stable business relationships with many well-known domestic companies as well as oversea customers. With the goal of providing high quality products to customers at low cots, we are committed to improving its capacities in research, development, manufacturing and management. We have honored to receive recognition from our customers. Till now we have passed ISO9001 in 2005 and ISO/TS16949 in 2008. Enterprises of quality of survival, the credibility of development for the purpose, sincerely welcome domestic and foreign businessmen to visit to discuss cooperation.
We are long-term partners, there is no disappointment every time, we hope to maintain this friendship later!