Quality Inspection for Hcv Genotype Assay - Macro & Micro-Test Sample Release Reagent – Macro & Micro-Test
Quality Inspection for Hcv Genotype Assay - Macro & Micro-Test Sample Release Reagent – Macro & Micro-Test Detail:
Product name
Macro & Micro-Test Sample Release Reagent
Certificate
CE, FDA, NMPA
Features
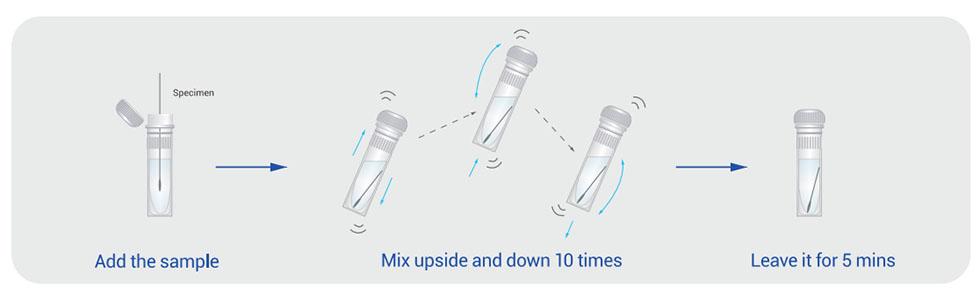
Rapid: only 5 mins.
Easy: only 3 steps (1.add the sample 2.Mix upside and down 10 times 3. Leave it for 5 mins).
Main components
| Name | Main components | Component specifications | Quantity |
| Sample Release reagent | Dithiothreitol, sodium dodecyl sulfate (SDS), RNase inhibitor, surfactant, purified water | 0.5mL/Vial | 50 Vial |
Note: Components in different batches of kits are not interchangeable.
Storage conditions and shelf life
Store and transport at room temperature. The shelf life is 24 months.
Applicable instruments
Instruments and equipment during sample processing, such as pipettes, vortex mixers, water baths, etc.
Sample requirements
Freshly collected oropharyngeal swabs, nasopharyngeal swabs.
Precision
When this kit is used for extraction from the in-house precision reference CV for 10 replicates, the coefficient of variation (CV, %) of Ct value is not more than 10%.
Inter-batch difference
When the in-house precision reference is tested on three batches of kits under trial production upon repeated extraction and, the coefficient of variation (CV, %) of Ct value is not more than 10%.
Performance comparison
● Extraction efficiency comparsion
|
Efficiency comparsion of magnetic beads method and sample releaser |
||||
|
concentration |
magnetic beads method |
sample releaser |
||
|
orfab |
N |
orfab |
N |
|
|
20000 |
28.01 |
28.76 |
28.6 |
29.15 |
|
2000 |
31.53 |
31.9 |
32.35 |
32.37 |
|
500 |
33.8 |
34 |
35.25 |
35.9 |
|
200 |
35.25 |
35.9 |
35.83 |
35.96 |
|
100 |
36.99 |
37.7 |
38.13 |
undet |
The extraction efficiency of sample releaser was similar to that of magnetic beads method, and the concentration of pathogen could be 200Copies/mL.
● CV value comparison
|
Repeatability of sample releaser extraction |
||
| concentration:5000Copies/mL |
ORF1ab |
N |
|
30.17 |
30.38 |
|
|
30.09 |
30.36 |
|
|
30.36 |
30.26 |
|
|
30.03 |
30.48 |
|
|
30.14 |
30.45 |
|
|
30.31 |
30.16 |
|
|
30.38 |
30.7 |
|
|
30.72 |
30.79 |
|
| CV |
0.73% |
0.69% |
When tested at 5,000 copies /mL, the CV of orFab and N was 0.73% and 0.69%, respectively.
Product detail pictures:


Related Product Guide:
Our firm sticks to the basic principle of Quality is the life of your company, and status will be the soul of it for Quality Inspection for Hcv Genotype Assay - Macro & Micro-Test Sample Release Reagent – Macro & Micro-Test , The product will supply to all over the world, such as: Rio de Janeiro, Uruguay, Bangladesh, Our items have national accreditation requirements for qualified, high quality goods, affordable value, was welcomed by people today all over the world. Our products will continue to enhance within the order and look forward to cooperation with you, Should really any of these products and solutions be of curiosity to you, be sure to letus know. We are likely to be content to offer you a quotation up on receipt of your detailed needs.
The product manager is a very hot and professional person, we have a pleasant conversation, and finally we reached a consensus agreement.