COVID-19, Flu A & Flu B Combo Kit
Product name
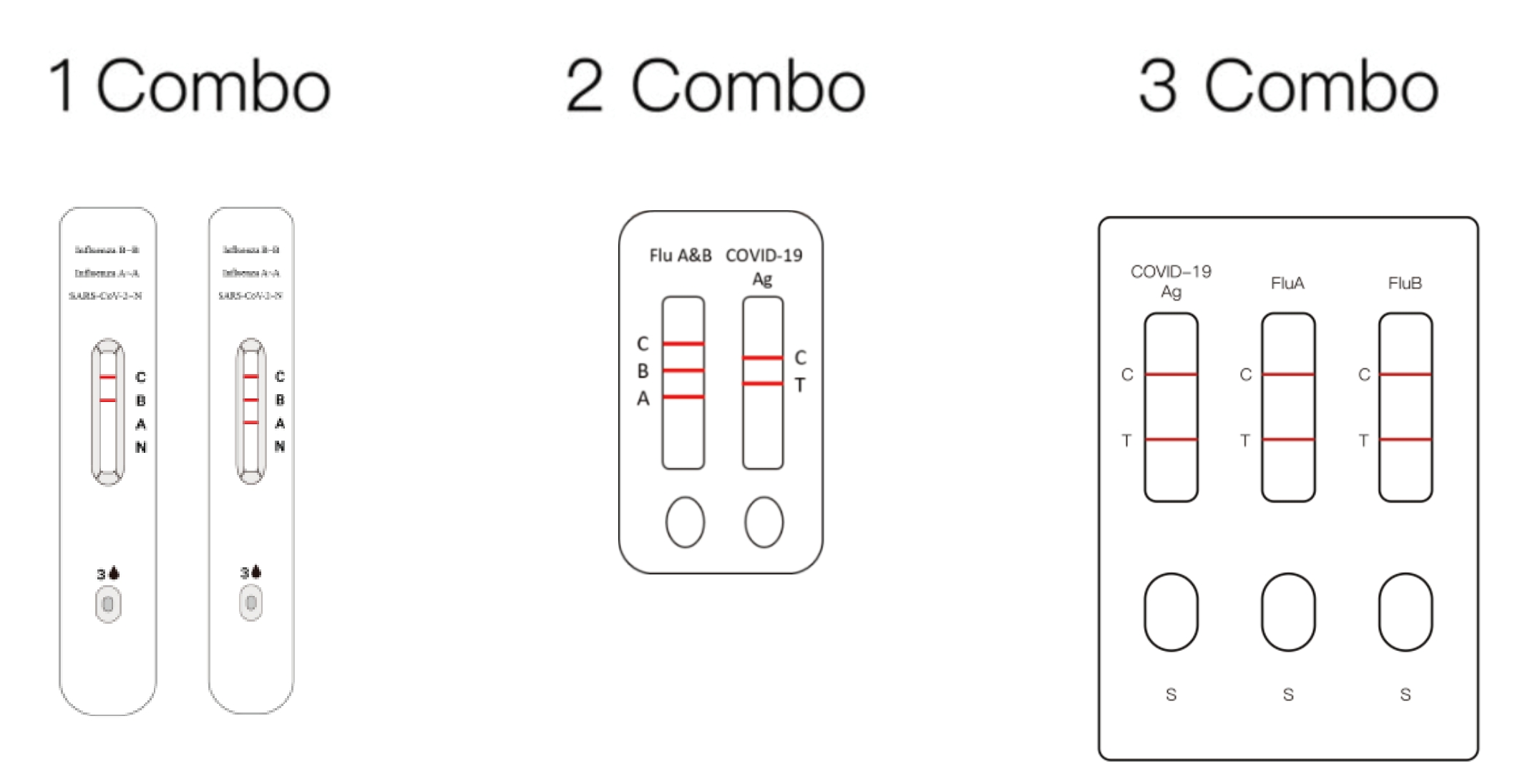
HWTS-RT098-SARS-COV-2 and Influenza A/B Antigen Detection Kit (Immunochromatography)
HWTS-RT101-SARS-COV-2, Influenza A&B Antigen Combined Detection Kit(Immunochromatography)
HWTS-RT096-SARS-COV-2, Influenza A and Influenza B Antigen Detection Kit(Immunochromatography)
Certificate
CE
Epidemiology
Coronavirus Disease 2019 (COVID-19), is a pneumonia caused by infection with a novel coronavirus named as Severe Acute Respiratory Syndrome Corona-Virus 2 (SARS-CoV-2). SARS-CoV-2 is a novel coronavirus in β genus, enveloped particles in round or oval, with a diameter from 60 nm to 140 nm. Human is generally susceptible to SARS-CoV-2. The main sources of infection are the confirmed COVID-19 patients and asymptomatic carrier of SARSCoV-2.
Influenza belongs to the orthomyxoviridae family and is a segmented negative strand RNA virus. According to the antigenicity difference of nucleocapsid protein (NP) and matrix protein (M), influenza viruses are divided into three types: A, B and C. Influenza viruses discovered in recent years will be classified as type D. Influenza A and influenza B are the main pathogens of human influenza, which have the characteristics of wide prevalence and strong infectivity. They can cause severe infection in children, the elderly and people with low immune function.
Technical Parameters
| Storage temperature | 4 - 30℃ at sealed and dry condition |
| Sample type | Nasopharyngeal swab、Oropharyngeal swab、Nasal swab |
| Shelf life | 24 months |
| Auxiliary instruments | Not required |
| Extra Consumables | Not required |
| Detection time | 15-20 mins |
| Specificity | There is no cross-reaction with pathogens such as Human coronavirus HCoV-OC43, HCoV-229E, HCoV-HKU1, HCoV-NL63, respiratory syncytial virus type A,B, parainfluenza virus type 1, 2, 3, rhinovirus A, B, C, adenovirus 1, 2, 3, 4, 5, 7,55,Chlamydia pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae,Neisseria meningitidis, Staphylococcus aureus, Streptococcus pneumoniae and other pathogens. |
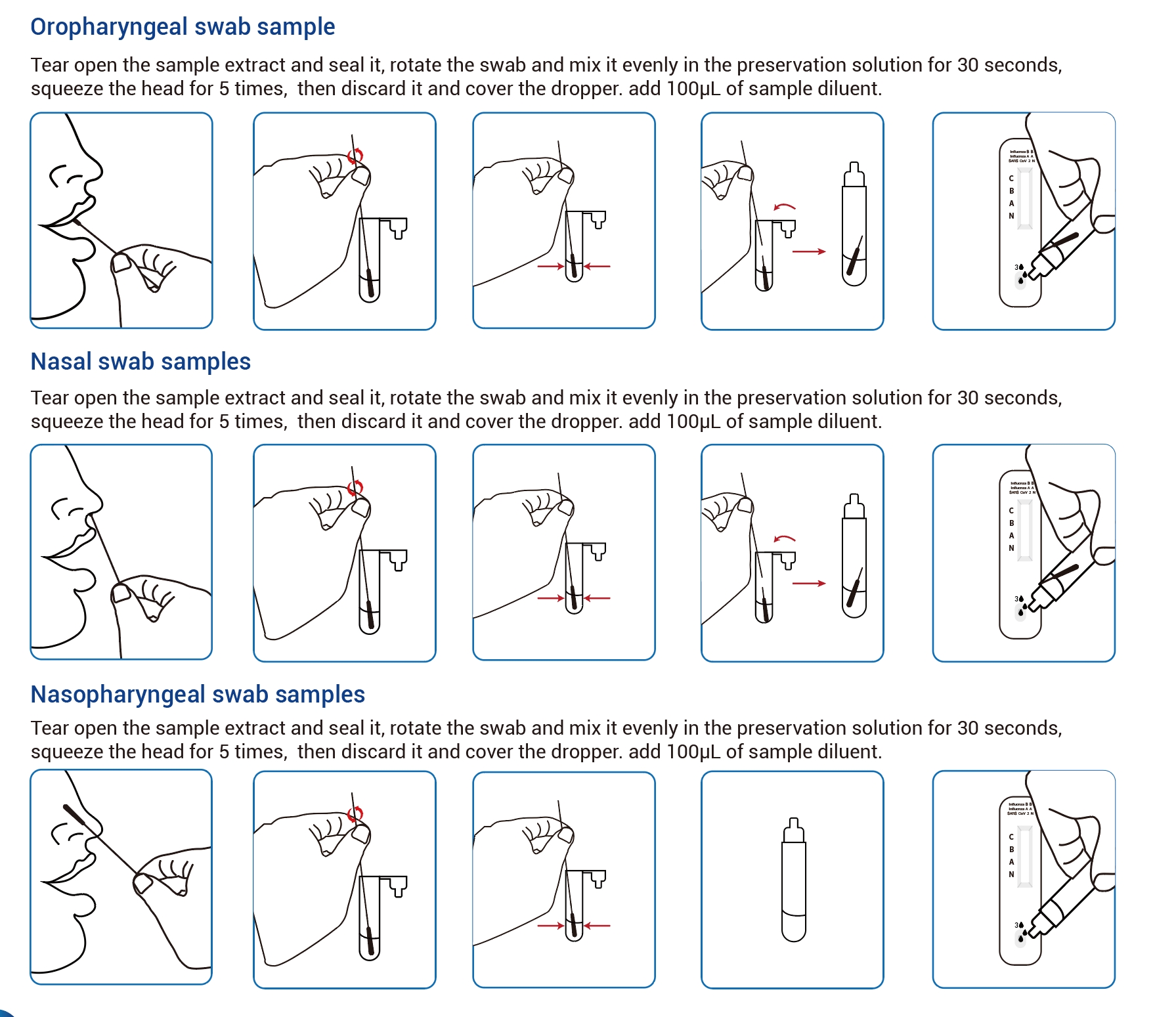
Work Flow
Main Components