Respiratory Pathogens Combined
Respiratory Pathogens Combined Detail:
Product name
HWTS-RT050-Six Kinds of Respiratory Pathogen Nucleic Acid Detection Kit (Fluorescence PCR)
Epidemiology
Influenza, commonly known as 'flu', is an acute respiratory infectious disease caused by influenza virus, which is highly infectious and is mainly transmitted by coughing and sneezing.
Respiratory syncytial virus (RSV) is an RNA virus, belonging to the paramyxoviridae family.
Human adenovirus (HAdV) is a double stranded DNA virus without envelope. At least 90 genotypes have been found, which can be divided into 7 subgenera A-G.
Human rhinovirus (HRV) is a member of the Picornaviridae family and the Enterovirus genus.
Mycoplasma pneumoniae (MP) is a pathogenic microorganism that is between bacteria and viruses in size.
Channel
| Channel | PCR-Mix A | PCR-Mix B |
| FAM Channel | IFV A | HAdV |
| VIC/HEX Channel | HRV | IFV B |
| CY5 Channel | RSV | MP |
| ROX Channel | Internal Control | Internal Control |
Technical Parameters
| Storage |
-18℃ |
| Shelf-life | 12 months |
| Specimen Type | Oropharyngeal swab |
| Ct | ≤35 |
| LoD | 500Copies/mL |
| Specificity | 1.The cross-reactivity test results showed that there was no cross reaction between the kit and human coronavirus SARSr-CoV, MERSr-CoV, HCoV-OC43, HCoV-229E, HCoV-HKU1, HCoV-NL63, Parainfluenza virus types 1, 2, and 3, Chlamydia pneumoniae, human metapneumovirus, Enterovirus A, B, C, D, Epstein-Barr virus, Measles virus, human cytomegalovirus, Rotavirus, Norovirus, Mumps virus, Varicella-zoster virus, Legionella, Bordetella pertussis, Haemophilus influenzae, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes, Klebsiella pneumoniae, Mycobacterium tuberculosis, Aspergillus fumigatus, Candida albicans, Candida glabrata, Pneumocystis jiroveci, Cryptococcus neoformans and human genomic nucleic acids.
2.Anti-interference ability: Mucin (60mg/mL), 10% (v/v) human blood, phenylephrine (2mg/mL), oxymetazoline (2mg/mL), sodium chloride (with preservatives) (20mg/mL), beclomethasone (20mg/mL), dexamethasone (20mg/mL), flunisolide (20μg/mL), triamcinolone acetonide (2mg/mL), budesonide (2mg/mL), mometasone ( 2mg/mL), Fluticasone (2mg/mL), histamine hydrochloride (5mg/mL), alpha-interferon (800IU/mL), zanamivir (20mg/mL), ribavirin (10mg/mL), oseltamivir (60ng/mL), peramivir (1mg/mL), lopinavir (500mg/mL), ritonavir (60mg/mL), mupirocin (20mg/mL), azithromycin (1mg/mL), cefprozil (40μg/mL), Meropenem (200mg/mL), levofloxacin (10μg/mL), and tobramycin (0.6mg/mL) were selected for the interference test, and the results showed that the interfering substances at the above concentrations had no interference reaction to the test results of pathogens. |
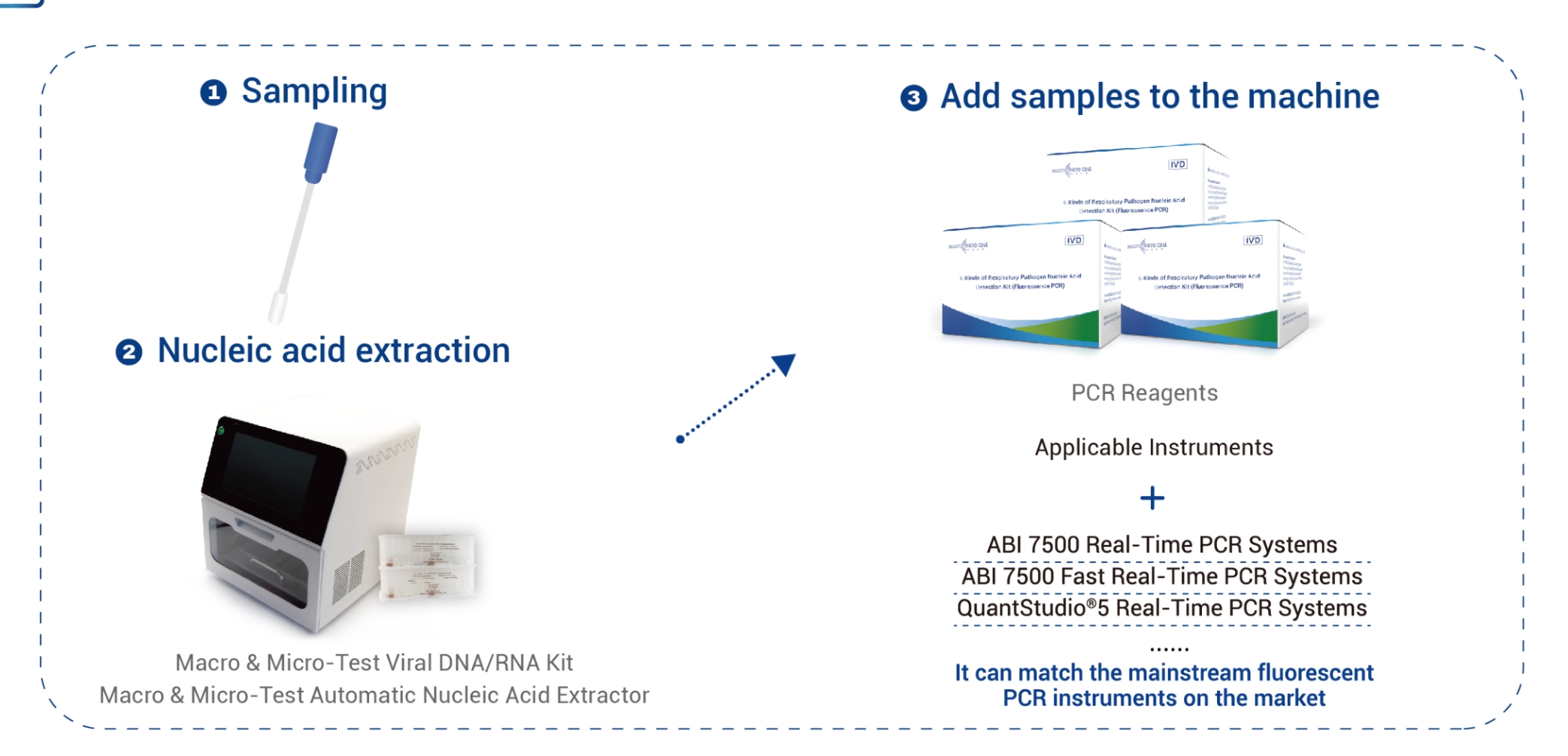
| Applicable Instruments | Applied Biosystems 7500 Real-Time PCR Systems
Applied Biosystems 7500 Fast Real-Time PCR Systems QuantStudio®5 Real-Time PCR Systems SLAN-96P Real-Time PCR Systems LightCycler®480 Real-Time PCR system LineGene 9600 Plus Real-Time PCR Detection Systems MA-6000 Real-Time Quantitative Thermal Cycler BioRad CFX96 Real-Time PCR System, BioRad CFX Opus 96 Real-Time PCR System |
Total PCR Solution
Product detail pictures:

Related Product Guide:
We not only will try our greatest to supply outstanding services to every shopper, but also are ready to receive any suggestion offered by our buyers for Respiratory Pathogens Combined , The product will supply to all over the world, such as: Tanzania, Czech Republic, Swansea, We welcome you to visit our company, factory and our showroom displayed various products that will meet your expectation, meanwhile, it is convenient to visit our website, our sales staff will try their efforts to offer you the best service. If you need to have more information, remember to do not hesitate to contact us by E-mail or telephone.
Product variety is complete, good quality and inexpensive, the delivery is fast and transport is security, very good, we are happy to cooperate with a reputable company!