SARS-CoV-2, Respiratory Syncytium, and Influenza A&B Antigen Combined
Product name
HWTS-RT152 SARS-CoV-2, Respiratory Syncytium, and Influenza A&B Antigen Combined Detection Kit (Latex Method)
Certificate
CE
Epidemiology
Novel coronavirus (2019, COVID-19), referred to as "COVID-19", refers to pneumonia caused by novel coronavirus (SARS-CoV-2) infection.
Respiratory syncytial virus (RSV) is a common cause of upper and lower respiratory tract infections, and it is also the main cause of bronchiolitis and pneumonia in infants.
According to the antigenicity difference between core-shell protein (NP) and matrix protein (M), influenza viruses are classified into three types: A, B and C. Influenza viruses discovered in recent years will be classified as D. Among them, A and B are the main pathogens of human influenza, which have the characteristics of wide epidemic and strong infectivity, causing serious infections and life-threatening in children, the elderly and people with low immune function.
Technical Parameters
|
Target region |
SARS-CoV-2, Respiratory Syncytium, Influenza A&B Antigen |
|
Storage temperature |
4-30 ℃ sealed and dry for storage |
|
Sample type |
Nasopharyngeal swab、Oropharyngeal swab、Nasal swab |
|
Shelf life |
24 months |
|
Auxiliary instruments |
Not required |
|
Extra Consumables |
Not required |
|
Detection time |
15-20 mins |
Work Flow
● Nasopharyngeal swab samples:
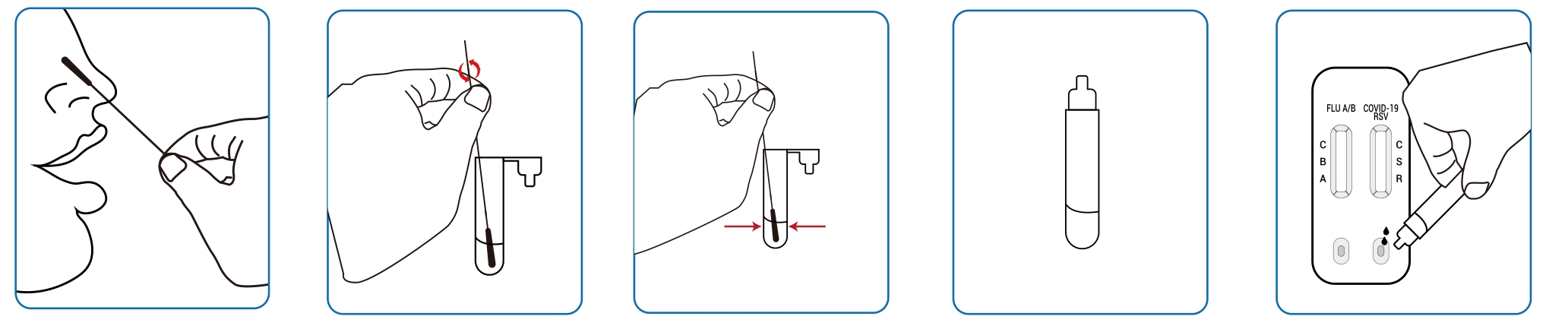
● Oropharyngeal swab sample:
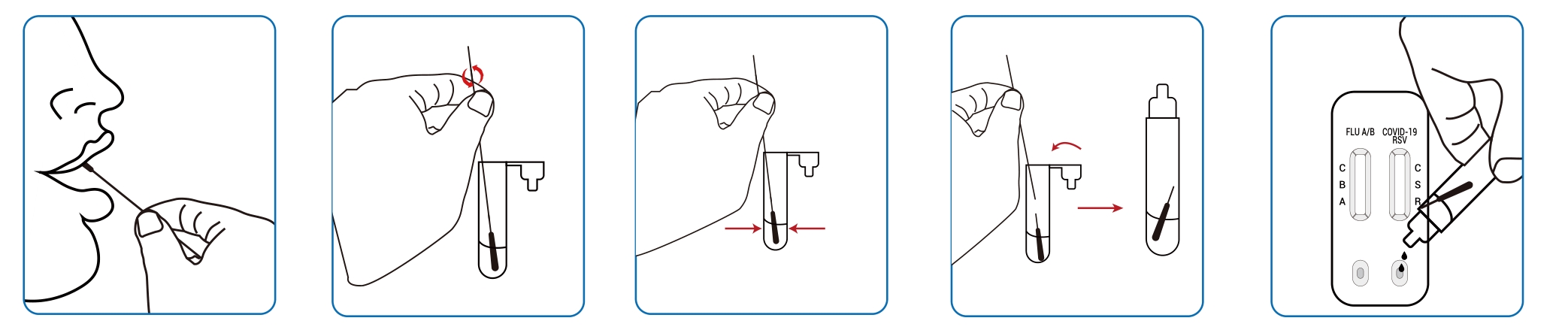
● Nasal swab samples:
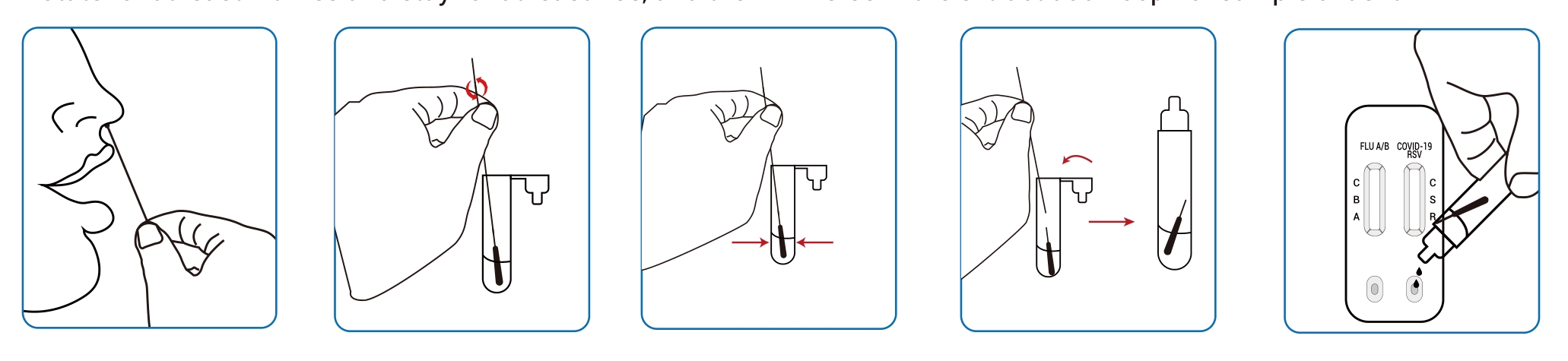
Precautions:
1. Do not read the result after 20 mins.
2. After opening, please use the product within 1 hour.
3. Please add samples and buffers in strict accordance with the instructions.