SARS-CoV-2/influenza A /influenza B
Product name
HWTS-RT148-SARS-CoV-2/influenza A /influenza B Nucleic Acid Combined Detection Kit (Fluorescence PCR)
Channel
| Channel Name | PCR-Mix 1 | PCR-Mix 2 |
| FAM Channel | ORF1ab gene | IVA |
| VIC/HEX Channel | Internal control | Internal control |
| CY5 Channel | N gene | / |
| ROX Channel | E gene | IVB |
Technical Parameters
| Storage |
-18℃ |
| Shelf-life | 12 months |
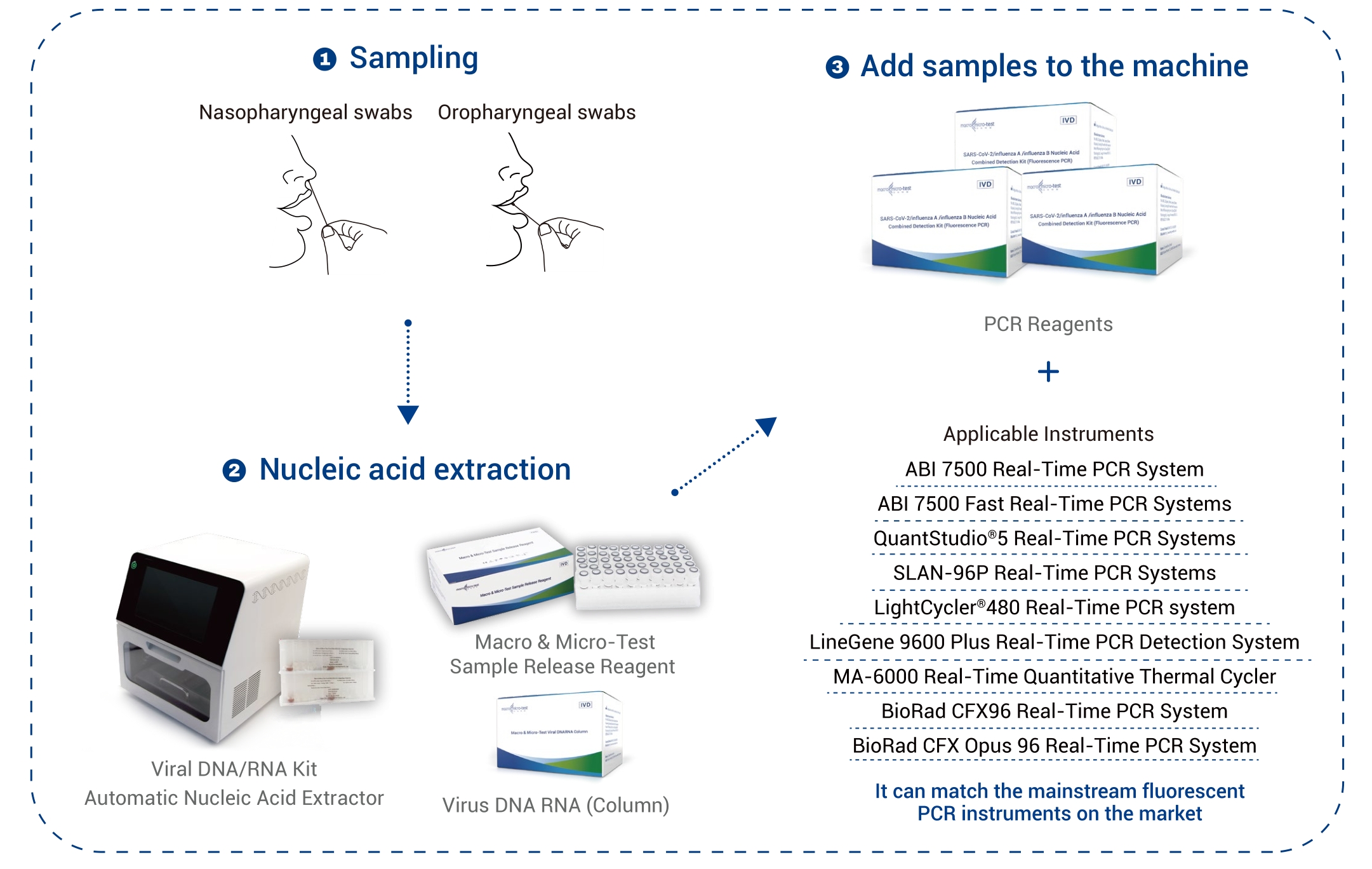
| Specimen Type | nasopharyngeal swabs and oropharyngeal swabs |
| Target | SARS-CoV-2 three targets ( Orf1ab, N and E genes )/influenza A /influenza B |
| Ct | ≤38 |
| CV | ≤10.0% |
| LoD | SARS-CoV-2:300 Copies/mL
influenza A virus:500 Copies/mL influenza B virus:500 Copies/mL |
| Specificity | a) The cross test results showed that the kit was compatible with human coronavirus SARSr- CoV, MERSr-CoV, HcoV-OC43, HcoV-229E, HcoV-HKU1, HCoV-NL63, respiratory syncytial virus A and B, parainfluenza virus 1, 2 and 3, rhinovirusA, B and C, adenovirus 1, 2, 3, 4, 5, 7 and 55, human metapneumovirus, enterovirus A, B, C and D, human cytoplasmic pulmonary virus, EB virus, measles virus Human cytomegalovirus, rotavirus, norovirus, mumps virus, varicella zoster virus, Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella, pertussis, Haemophilus influenzae, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes, Klebsiella pneumoniae, Mycobacterium tuberculosis, Aspergillus fumigatus, Candida albicans, Candida glabrata There was no cross reaction between Pneumocystis yersini and Cryptococcus neoformans.
b) Anti interference ability: select mucin (60mg/mL), 10% (V/ V) human blood, diphenylephrine (2mg/mL), hydroxymethylzoline (2mg/mL), sodium chloride (containing preservative) (20mg/mL), beclomethasone (20mg/mL), dexamethasone (20mg/mL), flunisone (20μg/mL), triamcinolone acetonide (2mg/mL), budesonide (2mg/mL), mometasone (2mg/mL), fluticasone (2mg/mL), histamine hydrochloride (5mg/mL), α-Interferon (800IU/mL), zanamivir (20mg/mL), ribavirin (10mg/mL), oseltamivir (60ng/mL), pramivir (1mg/mL), lopinavir (500mg/mL), ritonavir (60mg/mL), mupirocin (20mg/mL), azithromycin (1mg/mL), ceprotene (40μg/mL) Meropenem (200mg/mL), levofloxacin (10μg/mL) and tobramycin (0.6mg/mL). The results showed that the interfering substances at the above concentrations had no interference response to the detection results of pathogens. |
| Applicable Instruments | Applied Biosystems 7500 Real-Time PCR Systems
Applied Biosystems 7500 Fast Real-Time PCR Systems SLAN ® -96P Real-Time PCR Systems QuantStudio™ 5 Real-Time PCR Systems LightCycler® 480 Real-Time PCR system LineGene 9600 Plus Real-Time PCR Detection System MA-6000 Real-Time Quantitative Thermal Cycler BioRad CFX96 Real-Time PCR System BioRad CFX Opus 96 Real-Time PCR System |
Total PCR Solution