Well-designed Dengue Ns1 Antigen - Dengue Virus I/II/III/IV Nucleic Acid Detection Kit (Fluorescence PCR) – Macro & Micro-Test
Well-designed Dengue Ns1 Antigen - Dengue Virus I/II/III/IV Nucleic Acid Detection Kit (Fluorescence PCR) – Macro & Micro-Test Detail:
Product name
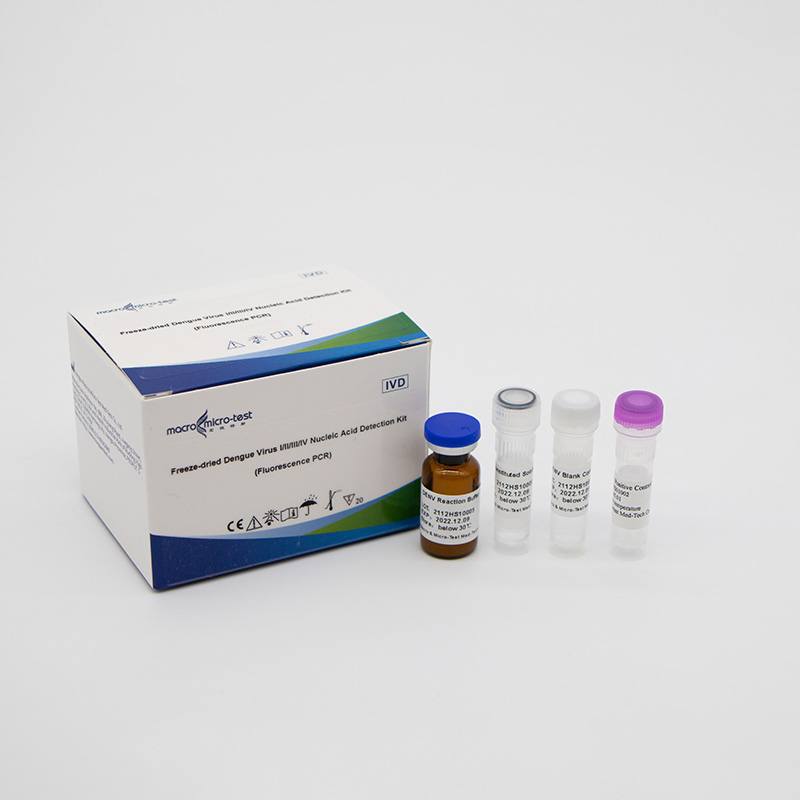
Dengue Virus I/II/III/IV Nucleic Acid Detection Kit (Fluorescence PCR)
Freeze-dried Dengue Virus I/II/III/IV Nucleic Acid Detection Kit (Fluorescence PCR)
Certificate
CE
Epidemiology
Dengue fever (DF), which is induced by denguevirus (DENV) infection, is one of the most epidemic arbovirus infectious diseases. DENV belongs to flavivirus under flaviviridae, and can be classified into 4 serotypes according to surface antigen. Its transmission medium includes Aedes aegypti and Aedes albopictus, prevalent mainly in tropical and subtropical areas.
Clinical manifestations of DENV infection mainly include headache, fever, weakness, enlargement of lymph node, leukopenia and etc., and bleeding, shock, hepatic injury or even death in severe cases. In recent years, climate change, urbanization, quick development of tourism and other factors have provided more rapid and convenient conditions for transmitting and spreading of DF, leading to constant expansion of epidemic area of DF.
Features
● Multiplex PCR Amplification Technology.
● Sub-Package: Safer and easier.
● High sensitivity: 500 Copies/mL.
● High specificity: No cross reactivity with common pathogens causing febrile and hemorrhagic diseases.
Channel
FAM: Dengue Virus I
VIC(HEX): Dengue Virus II
ROX: Dengue Virus III
CY5: Dengue Virus IV
PCR Amplification Conditions Setting
| Step | Cycles | Temperature | Time | Collect Fluorescent Signals or Not |
| 1 | 1 cycle | 50℃ | 30mins | No |
| 2 | 1 cycle | 95℃ | 15mins | No |
| 3 | 40 cycles | 95℃ | 15s | No |
| 60℃ | 45s | Yes |
Technical Parameters
| Storage | |
| Liquid | ≤-18℃ in dark; lyophilization:≤30℃ in dark |
| Shelf-life | 12 months |
| Specimen Type | Fresh Serum |
| Ct | ≤38 |
| CV | ≤5.0% |
| LoD | 500 Copies/mL |
Specificity: Perform cross reaction tests of Japanese encephalitis virus, Forest encephalitis virus, severe fever with thrombocytopenia syndrome, Xinjiang hemorrhagic fever, hantaan virus, hepatitis C virus, influenza A virus, influenza B virus and etc. No cross reaction is detected.
Applicable Instruments: It can match the mainstream fluorescent PCR instruments on the market.
SLAN-96P Real-Time PCR Systems.
ABI 7500 Real-Time PCR Systems.
ABI 7500 Fast Real-Time PCR Systems.
QuantStudio®5 Real-Time PCR Systems.
LightCycler®480 Real-Time PCR Systems.
LineGene 9600 Plus Real-Time PCR Detection Systems.
MA-6000 Real-Time Quantitative Thermal Cycler.
BioRad CFX96 Real-Time PCR System.
BioRad CFX Opus 96 Real-Time PCR System.
Main Components
Dengue Virus I/II/III/IV Nucleic Acid Detection Kit (Fluorescence PCR)
| Component | Catalogue Number | Quantity | Component Description | |
| HWTS-FE034A | HWTS-FE034B | |||
| Specification(20 tests/kit) | Specification(50 tests/kit) | |||
| DENV Reaction Buffer | 394μL/vial | 985μL/vial | 1 vial | Dengue virus I/II/III/IV primers, fluorescent probes, reaction buffer, Taq enzyme, reverse transcriptase and UDG enzyme, etc. |
| DENV Enzyme Mix | 6μL/vial | 15μL/vial | 1 vial | Reverse transcriptase, etc. |
| DENV Positive Control | 400μL/vial | 1 mL/vial | 1 vial | Mixture of DENV I/II/III/IV templates |
| DENV Blank Control | 400μL/vial | 1 mL/vial | 1 vial | DNase/RNase free H2O |
Freeze-dried Dengue Virus I/II/III/IV Nucleic Acid Detection Kit (Fluorescence PCR)
| Component | Character | Catalogue Number | Component Description | |
| HWTS-FE004B | HWTS-FE004C | |||
| Specification/Quantity(20 tests/kit) | Specification/Quantity (50 tests/kit) | |||
| DENV Reaction Buffer | lyophilized | 1 bottle | 1 bottle | dengue virus I/II/III/IV primers, fluorescent probes, reaction buffer, Taq enzyme, reverse transcriptase and UDG enzyme, etc. |
| DENV Positive Control | lyophilized | 1 vial | 1 vial | Mixture of DENV I/II/III/IV templates |
| DENV Blank Control | Liquid | 1 vial, 600μL/vial | 1 vial, 600μL/vial | DNase/RNase free H2O |
| Reconstituted Solution | Liquid | 1 vial, 1mL/vial | 1 vial, 1.45mL/vial | DNase/RNase free H2O, etc. |
Total PCR Solution

Reference
[1] Shu P Y, Chang S F, Kuo Y C, et al. Development of Group- and Serotype-Specific One-Step Sybr Green I-Based Real-Time Reverse Transcription-PCR Assay for Dengue Virus[J]. Journal of Clinical Microbiology, 2003, 41(6):2408-2416.
[2] Paudel D, Jarman R , Limkittikul K, et al. Comparison of real-time SYBR green dengue assay with real-time taqman RT-PCR dengue assay and the conventional nested PCR for diagnosis of primary and secondary dengue infection[J]. North American Journal of Medical Sciences, 2011, 3(10):478-485.
[3] Waggoner J J, Ballesteros G, Gresh L, et al. Clinical evaluation of a single-reaction real-time RT-PCR for pan-dengue and chikungunya virus detection[J]. Journal of Clinical Virology the Official Publication of the Pan American Society for Clinical Virology, 2016:57-61.
[4] Yao J X, Zhao W, Zhang L, et al. Research of Real-Time Fluorescence PCR Detection on Dengue Virus[J]. West China Medical Journal, 2009.
Product detail pictures:




Related Product Guide:
Our solutions are widely recognized and trusted by consumers and will meet up with constantly developing financial and social requires for Well-designed Dengue Ns1 Antigen - Dengue Virus I/II/III/IV Nucleic Acid Detection Kit (Fluorescence PCR) – Macro & Micro-Test , The product will supply to all over the world, such as: Iraq, Mecca, US, Due to the changing trends in this field, we involve ourselves into products trade with dedicated efforts and managerial excellence. We maintain timely delivery schedules, innovative designs, quality and transparency for our customers. Our moto is to deliver quality products within stipulated time.
The customer service staff's answer is very meticulous, the most important is that the product quality is very good, and packaged carefully, shipped quickly!